CLEARWATER, FL – MARCH 16, 2020 – Apyx™ Medical Corporation (NASDAQ:APYX) (the “Company”), a maker of medical devices and supplies and the developer of Helium Plasma Technology, marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market, today announced it will be restating its previously issued consolidated financial statements for the twelve months ended December, 31 2018 and the quarterly statements for the three and nine months ended September 30, 2018 and three months ended March 31, 2019. This decision was approved by the Company’s Board of Directors upon the recommendation of the Company’s Audit Committee, and after consultation with Management and the Company’s predecessor independent registered public accounting firm.
Investors should no longer rely upon the Company’s previously released financial statements for the time periods cited above. Similarly, related press releases, earnings releases, and investor communications describing the Company’s financial statements for these periods should no longer be relied upon.
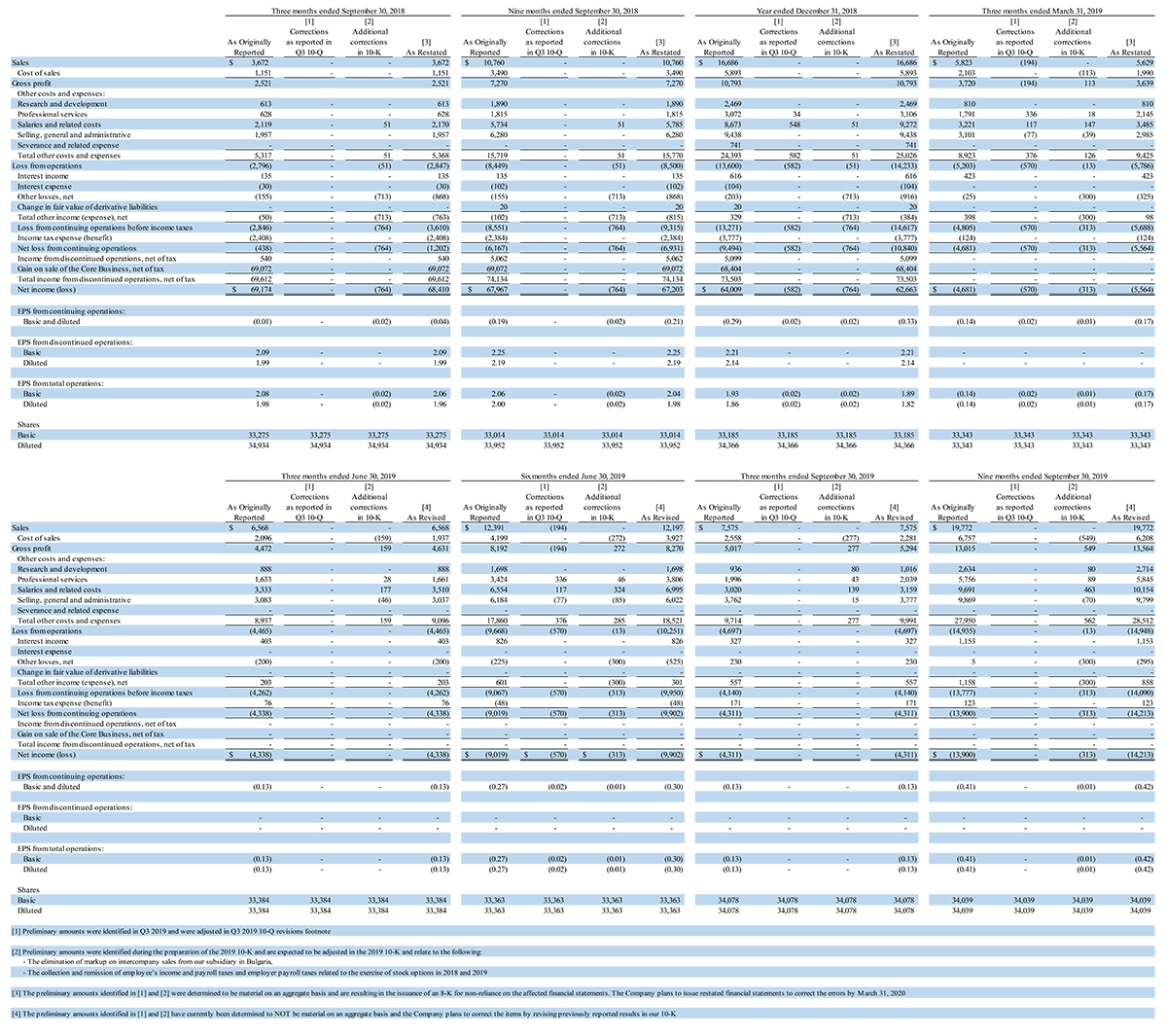
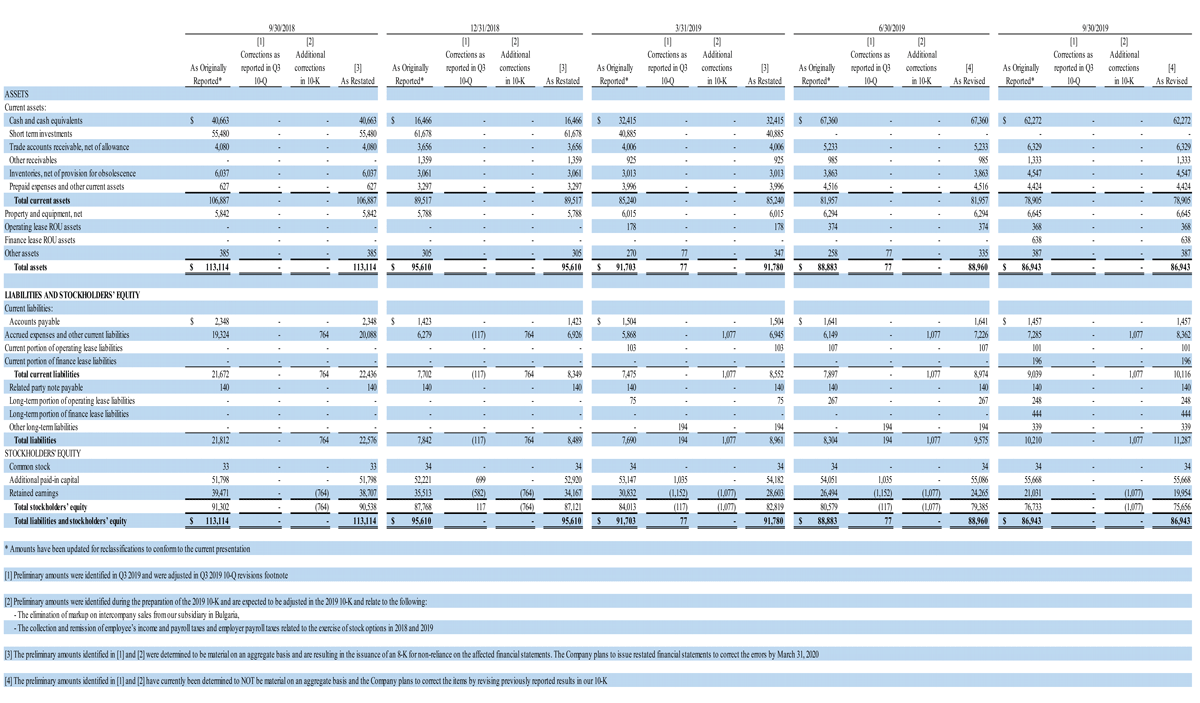
The decision to restate these financial statements is based on the conclusion that the financial statements for the aforementioned financial periods cannot be relied upon as a result of the aggregation of errors identified by management and the Company’s new accounting personnel during the preparation of its fiscal year 2019 Form 10-K and third quarter 2019 Form 10-Q, related to the following:
As identified during preparation of the fiscal year 2019 Form 10-K:
- The elimination of markup on intercompany sales from our subsidiary in Bulgaria
- For the three months ended March 31, 2019, the total impact included increases to both gross profit and to operating expenses of approximately $113,000.
- The collection and remission of employee’s income and payroll taxes related to the exercise of stock options in 2018 and 2019; the accrual and remission of the employer portion of payroll taxes related to those stock options exercises; reporting the incorrect amount of income to employees on their form W-2 for both non qualified and incentive stock option exercises, and misclassification of some non-qualified stock option exercises as incentive stock option exercises
- For the three and nine months ended September 30, 2018 and year ended December 31, 2018, the total aggregated impact included an increase of approximately $51,000 to operating expenses, an increase of approximately $713,000 to other losses and an increase to net loss of approximately $764,000.
- For the three months ended March 31, 2019, the total aggregated impact included an increase to operating expenses of $13,000, an increase of approximately $300,000 to other losses and an increase to net loss of approximately $313,000.
As previously disclosed and adjusted in Form 10-Q for the three and nine months ended September 2019 filed on November 11, 2019:
- Accounting for stock-based compensation expense (related to forfeitures, vesting periods, modifications, fair value measurements and other miscellaneous items)
- For the year ended December 31, 2018, the total impact included increases to operating expenses, operating loss and net loss of approximately $582,000 each.
- For the three months ended March 31, 2019, the total impact included increases to operating expenses, operating loss and net loss of approximately $453,000 each.
- Accounting for revenue and deferred expenses related to pre-development activities in some of our OEM contracts
- For the three months ended March 31, 2019, the total impact included decreases to sales of approximately $194,000, decreases to operating expenses of approximately $77,000 and increases to both operating loss and net loss of approximately $117,000.
A full reconciliation of these restatement adjustments, as well as revisions deemed to have an immaterial impact to the periods ended June 30 and September 30, 2019 is included with this press release and is available on the investor relations page of the Company’s website.
Apyx Medical has experienced a significant business transformation over the last couple of years, including the disposition of its Core business in 2018 and changes to key management personnel. Throughout 2019, the Company has been making efforts to remediate its material weaknesses in internal controls over financial reporting identified in 2018, including investing in new personnel that have expertise in a broad array of accounting topics. As a result of these investments and remediation efforts, these errors in reporting were identified during 2019. Due to the aggregation of these errors, the Company’s predecessor independent registered public accounting firm determined the changes were material enough to require a restatement of the prior periods aforementioned.
Today’s announcement reiterates the Company’s continued commitment to best practices and upholding the highest standards of financial reporting. Apyx Medical continues to be committed to building a strong foundation of processes, procedures, systems and talent in order to capitalize on future global growth opportunities.
About Apyx Medical Corporation
Apyx Medical Corporation (formerly Bovie Medical Corporation) is an advanced energy technology company with a passion for elevating people’s lives through innovative products in the cosmetic and surgical markets. Known for its innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients it serves. The company’s Helium Plasma Technology is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers plastic surgeons, fascial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision and virtually eliminating unintended tissue trauma. The Company also leverages its deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Form 10-K for the year ended December 31, 2019. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Westwicke Partners on behalf of Apyx Medical Corporation
Mike Piccinino, CFA
investor.relations@apyxmedical.com
PDF Link – Reconciliation of Restatement Adjustments and Revisions: http://apyxmedical.com/wp-content/uploads/2020/03/apyx_supplemental-reconciliation-of-restatement-adjustments-and-revisions_march-16_2020.pdf