BOVIE MEDICAL CORPORATION TO PARTICIPATE IN DREXEL HAMILTON MICRO-CAP INVESTOR FORUM
CLEARWATER, FL — November 9, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that Jay Ewers, Chief Financial Officer of the Company, and Todd Hornsby, Vice President of Sales, will participate in the Drexel Hamilton Micro Cap Investor Forum.
The forum will be held at the Drexel Hamilton offices in New York. Mr. Ewers and Mr. Hornsby will present at 3:30pm on November 12, 2015. Investors attending may arrange a one-on-one meeting by contacting Drexel Hamilton.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Investor Relations:
MBS Value Partners
Hugh Collins
(212) 223-4632
investor.relations@boviemed.com
- Total Sales increased 15.3% year-on-year; Gross margin was 41.3%
- J-Plasma sales of $505,000 exceeded total sales for first half 2015
- Expanded Medical Advisory Board with world renowned leaders in the fields of cardiovascular and cardiothoracic surgery
- Strengthened supply chain and product development efficiencies with purchase of R&D and Manufacturing Contractor in Bulgaria in mid-October
J-Plasma® Operating Metrics
- Generators in use increased 82% to 62, up from 34 at end of Q2
- 79 Scrub Purchase Orders, almost double the 42 for first half 2015
- Systems approved by 59 Hospital Value Analysis Committees (VACs), up from 42 at end of Q2; under review by 64 additional VACs
CLEARWATER, FL — NOV 4, 2015- Bovie Medical Corporation (NYSEMKT:BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced results for the third quarter ended September 30,2015.
Management Comments
“The third quarter marked an inflection point for Bovie Medical,” said Robert L. Gershon, Chief Executive Officer. “The steady build-up in surgeon adoption of J-Plasma® was reflected in major sequential increases across all of our commercialization metrics, from Scrub Purchase Orders through Generators in Use. This strong momentum resulted in third quarter J-Plasma® sales that exceeded the aggregate sales for the first two quarters of 2015. At the same time, our core business revenues increased in line with our expectation for higher electro-generator sales in the second half of 2015, and continued to provide important infrastructure support for the J-Plasma® roll-out.”
“The leading indicators for J-Plasma® have reached a level that we expect should yield strong sales momentum in the coming periods. With 62 generators in use, we have the scale we need to increase adoption within our current installed base and move forward to broaden adoption within those hospitals where J-Plasma® is VAC-approved, while expanding the universe of surgeons who are using the product. By the end of the third quarter there were 132 surgeons using J-Plasma®, a significant increase from the 76 surgeons who were using the product three months earlier.
“J-Plasma® is increasingly being recognized by top surgeons across a range of specialties for its precision and effectiveness. Last quarter we announced the formation of a Medical Advisory Board to assist us in executing on a multi-specialty J-Plasma® strategy, and we named Dr. Vip Patel, a world renowned urologist and leader in robotic surgery as its first member. In the third quarter, we added two equally impressive members: Dr. Husam H. Balkhy, a pioneer in the field of minimally invasive and robotic cardiac surgery and Dr. Robert J. Cerfolio, an internationally recognized expert in robotic cardiothoracic surgery. Expansion of J-Plasma® target specialties to urology, cardiovascular and cardio thoracic surgery under the guidance of these well-recognized thought leaders should significantly increase the size of J-Plasma®’s addressable market. Additionally, this strategy should accelerate the hospital VAC approval process, which continues to extend our sales cycle.”
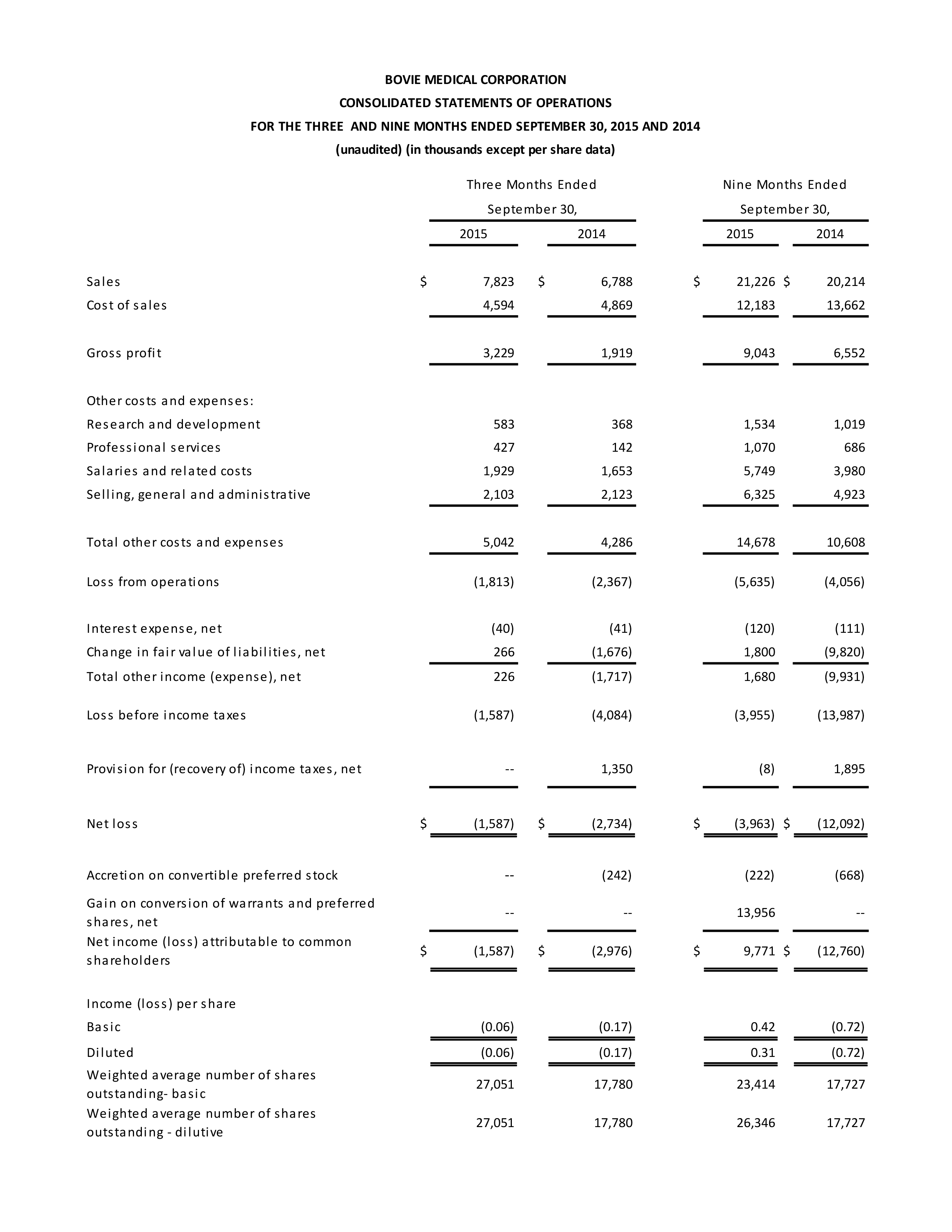
Third Quarter 2015 Results
Third quarter sales were $7.8 million, up 15.3% from $6.8 million in the third quarter of 2014 on higher sales of core, OEM, and J-Plasma® products. Gross margin was 41.3%, stable with last year’s third quarter adjusted gross margin of 41.3%. In last year’s third quarter the Company reported gross margin of 28.3%, which included costs related to excess and obsolete inventories and other adjustments.
Operating expenses totaled $5.0 million in the third quarter, compared to $4.3 million in the third quarter of 2014. Approximately 80% of the year-on-year increase in operating expenses represented additional spending on R&D and the commercialization of J-Plasma®; SG&A remained stable both sequentially and year-on-year, as higher sales commissions were offset by administrative cost reductions. The Company’s operating loss was $1.8 million, compared to an operating loss of $2.4 million in the third quarter of 2014.
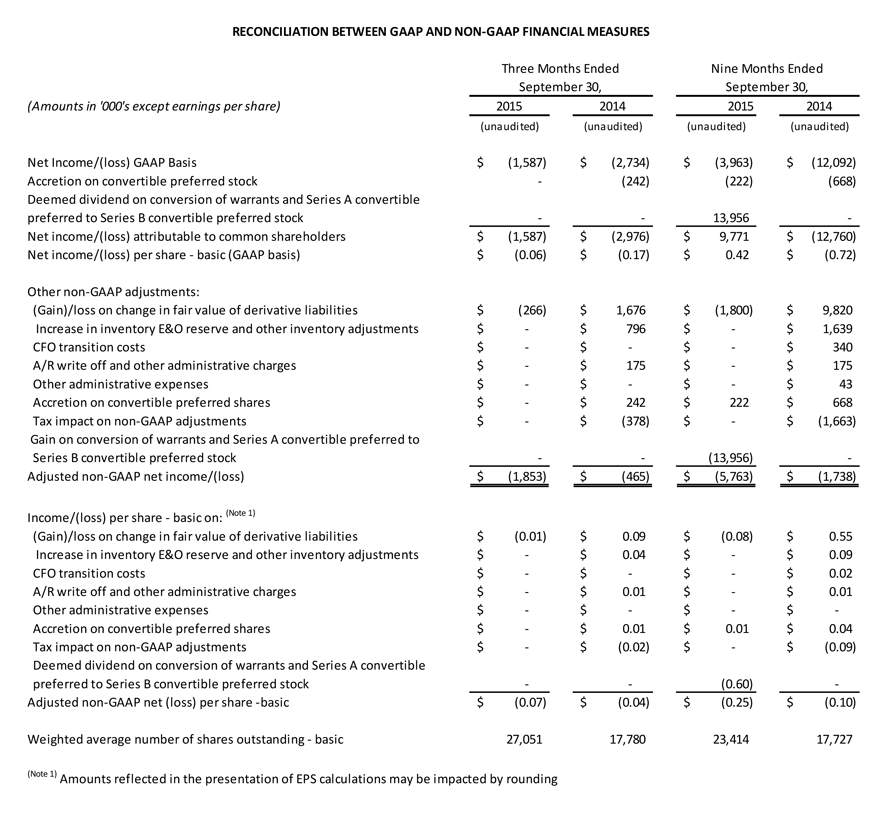
GAAP net loss attributable to common shareholders for the third quarter was $1.6 million, or $0.06 per diluted share, compared with GAAP net loss attributable to common shareholders of $3.0 million, or $0.17 per diluted share in the third quarter of 2014.
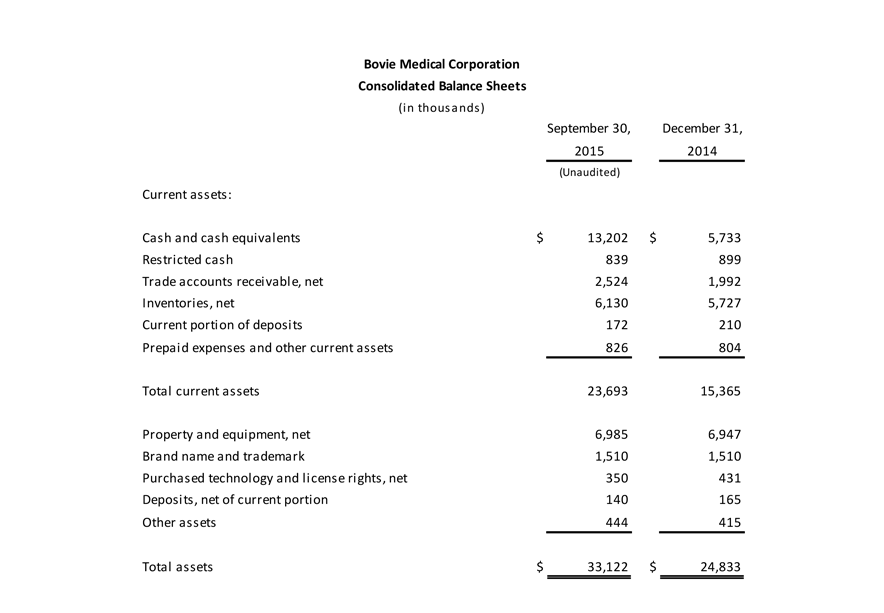
The Company had unrestricted cash and cash and equivalents of $13.2 million at the end of the third quarter, compared to $14.1 million at the end of this year’s second quarter.
Recent Developments
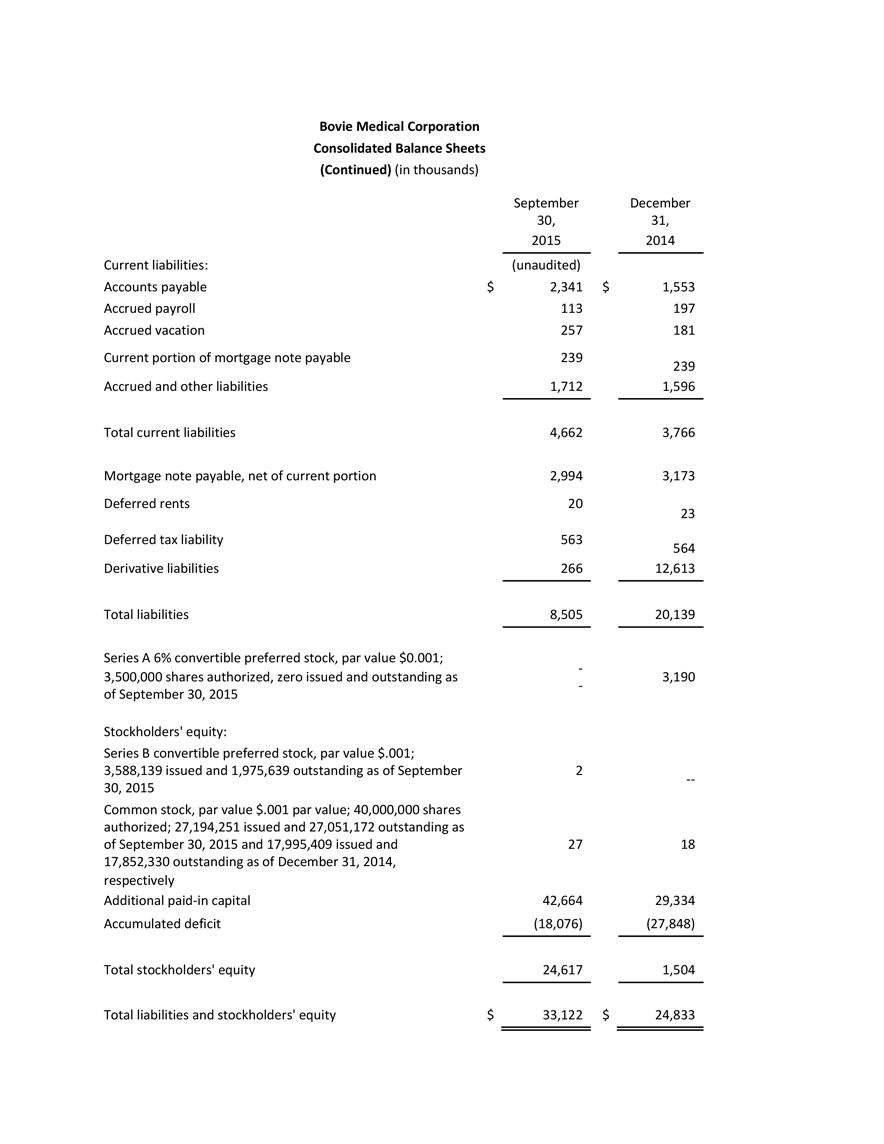
- Bovie announced the purchase of its Bulgarian R&D and manufacturing contractor (“Bovie Bulgaria”) for approximately $566,000 in cash. Bovie Bulgaria operates a 16,000 square foot ISO13485 certified and FDA registered manufacturing facility located in the capital city of Sofia, which houses manufacturing, development and assembly operations. The transaction gives Bovie full control over this important part of its product development and supply chain, particularly with respect to R&D for advanced surgical energy technology including key components of its J-Plasma® product line.
- Bovie received FDA 510(k) clearance for six new hand piece configurations that expand its J-Plasma® pistol grip portfolio, by expanding the range of instrument lengths and adding a new needle configuration to the standard blade configuration.
- The Bovie® Ultimate™ Operating Room Generator was named an “Innovation of the Year” by The Society of Laparoendoscopic Surgeons (SLS) on September 2, 2015.
- Bovie signed its first group purchasing organization (GPO) agreement with Amerinet, one of the nation’s largest healthcare GPOs, for the use of J-Plasma® by its members. Amerinet has more than 85,000 members, including 33,000 clinics, 3,500 acute care hospitals, and 3,300 ambulatory surgery centers.
- Jay Ewers was named Chief Financial Officer. Mr. Ewers has served as interim CFO since mid-June of this year and was previously the Company’s Corporate Controller.
The Medical Advisory Board
In the second quarter of 2015, Bovie established a Medical Advisory Board that will consist of surgeons who are thought-leaders in a range of different specialties and named Dr. Vipul Patel, a world renowned urologist and leader in robotic surgery, as its first member. Ultimately, the Board is expected to have 6 to 9 members and lead the usage of J-Plasma® for specific procedures within new target specialties including, but not limited to, cardiovascular, cardiothoracic and urology.
In the third quarter, the Board was increased to 3 members with the addition of two world-class surgeons:
- Dr. Husam H. Balkhy, is Director of Robotic and Minimally Invasive and Cardiac Surgery, and Associate Professor of Surgery at The University of Chicago Medicine. Recognized as a pioneer in the field, he specializes in cardiac diseases, using robotic and less invasive techniques to reduce pain, disability, and recovery time.
- Dr. Robert J. Cerfolio is an internationally recognized expert in robotic thoracic surgery. He received the James H. Estes Family Lung Cancer Research Endowed Chair in 2010, and Dr. Cerfolio is currently Professor of Surgery and Section Chief of Thoracic Surgery in the Division of Cardiothoracic Surgery, at the University of Alabama Hospital in Birmingham.
“We are honored that Drs. Balkhy and Cerfolio have agreed to join our Medical Advisory Board and assist us in bringing the significant surgical advantages of J-Plasma® to the appropriate procedures within their areas of expertise” Mr. Gershon noted.
“Accuracy and safety is paramount for all surgeons,” said Dr. Balkhy. “In cardiac surgery, J-Plasma® has the potential not only to improve accuracy and safety but will allow surgeons to operate with even more precision. J-Plasma® promises to be a very strong addition to the armamentarium of the heart surgeon.”
“J-Plasma® is potentially a revolutionary instrument and represents a paradigm shift in thoracic surgery,” said Dr. Cerfolio. “J-Plasma®’s combination of power and the ability to penetrate tissue less deeply means it could play a pivotal role in teaching younger surgeons and residents, and it may soon be a must-have in the armamentarium of any thoracic surgeon.”Summary and Outlook
“Our third quarter results represented significant progress in J-Plasma® sales, substantial momentum in those metrics that provide a roadmap for future J-Plasma® sales, and continued growth in our core business. This performance has laid the foundation for further positive revenue comparisons in both J-Plasma® and our core business this year, as we develop the pipeline that we have built over the last twelve months.
“Within this framework we remain diligent in managing our administrative and other non-revenue generating costs as well as our cash position, while investing in those areas that have the potential to drive future growth. We expect to achieve additional operating expense savings in 2016 and 2017 related to the purchase of our R&D and manufacturing contractor, Bovie Bulgaria, which we announced in mid-October.
“Looking to 2016, we expect our revenue performance to benefit from further sequential increases in J-Plasma® operating metrics in our initial target specialties and a continuation of steady progress in our core business. Additionally, we are working closely with the members of our Medical Advisory Board to identify and appropriately target those procedures for which J-Plasma® can become the standard of care within the high profile urology, cardiovascular and cardio thoracic surgical specialties, as well as further advancing its visibility within our immediate target specialty of gynecology. Importantly, we have a transformational product with an excellent track record, the right surgeon advocates, a direct sales force with multi-specialty experience, and the resources to capture a greater share of an expanded market,” Mr. Gershon concluded.
Conference Call Details
The Company’s management will host a conference call on Thursday, November 5, 2015 at 8:30am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx151105
A replay of the call will be available approximately one hour after the end of the call through February 5, 2016. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10075038.
Investor Relations Contact
MBS Value Partners
Lynn Morgen and Hugh Collins
212.750.5800
investor.relations@boviemed.com
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-K and 10-Q for the year ended December 31, 2014 and the quarters ended March 31, 2015 and June 30, 2015, respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
CLEARWATER, FL — October 23, 2015 – Bovie Medical (BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced that it will issue its financial results for the third quarter ended September 30, 2015 on Wednesday November 4, 2015 after market close.
Management will host a conference call on Thursday November 5, 2015 at 8:30am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx151105
A replay of the call will be available approximately one hour after the end of the call through February 5, 2016. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10075038.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Investor Relations:
MBS Value Partners
Lynn Morgen and Hugh Collins
212.750.5800
investor.relations@boviemed.com
- Significantly strengthens supply chain and product development efficiencies
- Operating synergies expected to generate net cost savings in 2016 and 2017 aggregating approximately $850,000
CLEARWATER, FL — October 20, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE MKT: BVX) a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it purchased its Bulgarian R&D and manufacturing contractor (“Bovie Bulgaria”) for approximately €500,000 ($570,000) in cash, payable over a five-year period. The initial cash payment of €375,000 ($427,000) was made at closing, and the remaining cash payment of approximately €125,000 ($143,000) is due on Oct.20, 2020.
Bovie Bulgaria operates a 16,000 square foot ISO13485 certified and FDA registered manufacturing facility located in the capital city of Sofia, which houses manufacturing, development and assembly operations. The transaction will ensure that Bovie has full control over this important part of its product development and supply chain, particularly with respect to R&D for advanced surgical energy technology including key components of its J-Plasma® product line. Additionally, the transaction will drive operating efficiencies that are expected to reduce Company operating expenses in 2016 and 2017 by an aggregate net amount of approximately $850,000.
Commenting on the purchase, Robert L. Gershon, Bovie’s Chief Executive Officer, noted, “We are pleased to have completed a transaction that brings in-house the technical know-how that Bovie Bulgaria has accumulated over the last 16 years in working with Bovie Medical and enables us to form a lasting partnership with its Managing Director, Nikolay Shilev. Nikolay has entered into a five-year employment contract with Bovie Bulgaria and will receive an inducement grant of 225,922 shares of Bovie Medical restricted stock priced at $2.00 per share that will vest ratably over a five-year period. The Bulgarian manufacturing facility will be fully dedicated to our production needs and will be of immediate financial benefit to the Company. Additionally, it positions us to capture more European business for both J-Plasma® and our core product portfolio.”
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-Q for the quarters ended June 30, 2015, and March 31, 2015 respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations:
MBS Value Partners
Hugh Collins/Jane Searle
212-223-4632
investor.relations@boviemed.com
CLEARWATER, FL — October 14, 2015 – Bovie Medical Corporation (“Bovie or the “Company”) (NYSE:BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it has named Jay Ewers to the position of Chief Financial Officer.
Mr. Ewers has served as interim CFO since mid-June of this year and was previously the Company’s Corporate Controller. He has more than 30 years of senior experience, having held financial executive positions in corporations ranging from early stage to high profile public companies with global operations in the medical equipment, manufacturing and semiconductor industries.
Commenting on the appointment, Robert L. Gershon, Chief Executive Officer said, “Jay’s performance in the role of interim CFO over these last three months has been outstanding, and it is with great pleasure that we announce his promotion to CFO. Jay has impressed our Board, our executives and staff members and our investors by his hands-on experience in financial planning management, internal controls and his grasp of the infrastructure we need to support future growth.”
Mr. Ewers noted, “I am pleased to take on permanent CFO responsibilities at Bovie during this exciting time in the Company’s history, when adoption of our J-Plasma® product continues to build in targeted and new specialties, and we add new products to our core business portfolio.”
Mr. Ewers is a certified public accountant, a certified internal auditor, and holds a BS in Accounting.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-Q for the quarters ended June 30, 2015, and March 31, 2015 respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations:
MBS Value Partners
Hugh Collins, 212-223-4632
investor.relations@boviemed.com
CLEARWATER, FL — September 24, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX) a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it has signed its first group purchasing organization (GPO) agreement with Amerinet for the use of J-Plasma by its members.
The 3-year agreement with Amerinet, one of the nation’s largest healthcare GPOs, is in effect now through September 2018. Amerinet has more than 85,000 members, including 33,000 clinics, 3,500 acute care hospitals, and 3,300 ambulatory surgery centers. Through this agreement, Amerinet members will have access to J-Plasma®, Bovie’s transformational surgical product, which enables surgeons to operate with greater precision and minimal thermal spread.
“This agreement represents an important step in advancing the adoption of J-Plasma, and we are delighted to partner with Amerinet, which has an excellent reputation for providing its members with high quality healthcare products at reasonable costs,” said Robert L. Gershon, Bovie’s Chief Executive Officer.
About Amerinet
As a leading national healthcare solutions organization, Amerinet collaborates with acute and non-acute care providers to create and deliver unique supply chain solutions through performance improvement resources, guidance and ongoing support. With better product standardization and utilization, new financial tools beyond contracting and alliances that help lower costs, raise revenue and champion quality, Amerinet enriches healthcare delivery for its members and the communities they serve. To learn more about how Amerinet can help you successfully navigate the future of healthcare, visit www.amerinet-gpo.com.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Media contact:
Rachel Kessler
646.863.3079/ 917.660.0608
Kessler.Rachel@Gmail.com
CLEARWATER, FL — September 10, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX) a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that Robert L. Gershon, Chief Executive Officer of the Company, will present at the 6th Annual Craig-Hallum Alpha Select Conference.
Mr. Gershon will present at noon on Thursday, September 17, 2015, at the Convene Conference Center in New York. During his presentation, Mr. Gershon will discuss the latest developments for J-Plasma®, Bovie’s transformational surgical product, which enables surgeons to operate with greater precision on and around delicate structures with minimal thermal spread. The presentation will be webcast at http://boviemedical.wpengine.com/financials.asp
Bovie Medical’s management team will also conduct one-on-one meetings. Interested investors attending the conference may arrange a meeting by contacting their Craig-Hallum representative
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma®, utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Investor Relations Contact
MBS Value Partners
Hugh Collins
212-223-4632
investor.relations@boviemed.com
BOVIE® ULTIMATE™ GENERATOR TO RECEIVE “INNOVATION OF THE YEAR” RECOGNITION
FROM THE SOCIETY OF LAPAROENDOSCOPIC SURGEONS (SLS)
–Generator offers monopolar, bipolar and plasma features-
-Second consecutive year that a Bovie product have been recognized for innovation by the SLS–
CLEARWATER, FL — September 2, 2015 – BovieMedical (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced that the Bovie® Ultimate™ Operating Room Generator has been named an “Innovation of the Year” by The Society of Laparoendoscopic Surgeons (SLS).
The Bovie® Ultimate™ is a high frequency electrosurgical generator that can be used for delivery of RF energy and/or helium gas plasma to cut, coagulate and ablate soft tissue during open and laparoscopic surgical procedures. The generator offers users monopolar, bipolar and plasma features in a single generator. It also has a helium-gas outlet, making it compatible with J-Plasma®, Bovie’s transformational helium ionization tool for cutting and coagulation.
This marks the second consecutive year that a Bovie Medical product has been recognized for innovation; in 2014, J-Plasma® won this prestigious recognition.
Commenting on this achievement, Mr. Robert L. Gershon, Chief Executive Officer said, “The Bovie® Ultimate™ is a leading-edge product that combines J-Plasma® technology with the highest wattage operating room electrosurgical generator, and as such has the potential to become the new gold standard for hospital-based use. It is specifically designed to give medical practitioners greater flexibility, including the ability to easily incorporate our J-Plasma® product, which provides increased access and precision in a range of surgical procedures. We appreciate this recognition from The Society of Laparoendoscopic Surgeons and are proud that once again one of Bovie’s products has been recognized for its innovation.”
The Society of Laparoendoscopic Surgeons (SLS) is an educational, non-profit organization established to ensure the highest standards for the practice of laparoscopic, endoscopic and minimally invasive surgery. Each year, SLS recognizes the most innovative products of the past year that have a multidisciplinary application in minimally invasive surgery. The Bovie® Ultimate™ is one of 7 products to receive this recognition in 2015.
Bovie Medical will receive the recognition at the opening ceremony of SLS’ Minimally Invasive Surgery Week Annual Meeting and Endo Expo this evening in New York City.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
About SLS
The Society of Laparoendoscopic Surgeons was established as an educational, non-profit organization to help ensure the highest standards for the practice of laparoscopic, endoscopic and minimally invasive surgery. The Society serves surgeons from various specialties and other health professionals who are interested in advancing their expertise in the diagnostic and therapeutic uses of Laparoendoscopic and minimally invasive surgical techniques. With an international membership of over 6,000 surgeons, the organization offers a unique approach to the study and education of minimally invasive surgery by bringing together different medical specialties that use the techniques and tools of minimally invasive surgery.
Investor Relations Contact
MBS Value Partners
Hugh Collins/Jane Searle
212-223-4632
investor.relations@boviemed.com
BOVIE MEDICAL RECEIVES FDA 510(k) APPROVAL FOR NEW J-PLASMA® PISTOL GRIP CONFIGURATIONS
-New configurations offer surgeons even greater access and precision-
CLEARWATER, FL — August 31, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it has received FDA 510(k) approval for six new hand piece configurations that expand its J-Plasma® pistol grip portfolio.
The pistol grip hand piece will now be available with instrument lengths of 15cm, 33cm and 45cm, in addition to the existing length of 27cm. Also, these new length hand pieces will each be available with a new needle configuration, as well as the standard blade configuration.
The new configurations have received FDA 510(k) approval and will be available beginning September 2015
“In commercializing J-Plasma®, we have implemented a voice of customer initiative that resulted in new designs to respond to specific surgical needs,” said Robert L. Gershon, Chief Executive Officer. “The new lengths of our pistol grip hand pieces offer surgeons greater access and increases the opportunity for adoption across multiple specialties, while the new needle tip configuration provides even more precision resulting in less collateral damage to unintended tissue.”
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-Q for the quarters ended June 30, 2015, and March 31, 2015 respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
MBS Value Partners
Hugh Collins/Jane Searle
212-223-4632
investor.relations@boviemed.com
CLEARWATER, FL — August 25, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that Robert L. Gershon, Chief Executive Officer of the Company, will participate in the Barrington Research 8th Annual Fall Investment Conference.
The conference will be held at the Four Seasons Hotel in Chicago, and Mr. Gershon will present to investors on September 1, 2015. Investors attending may arrange a one-on-one meeting by contacting Barrington Research.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
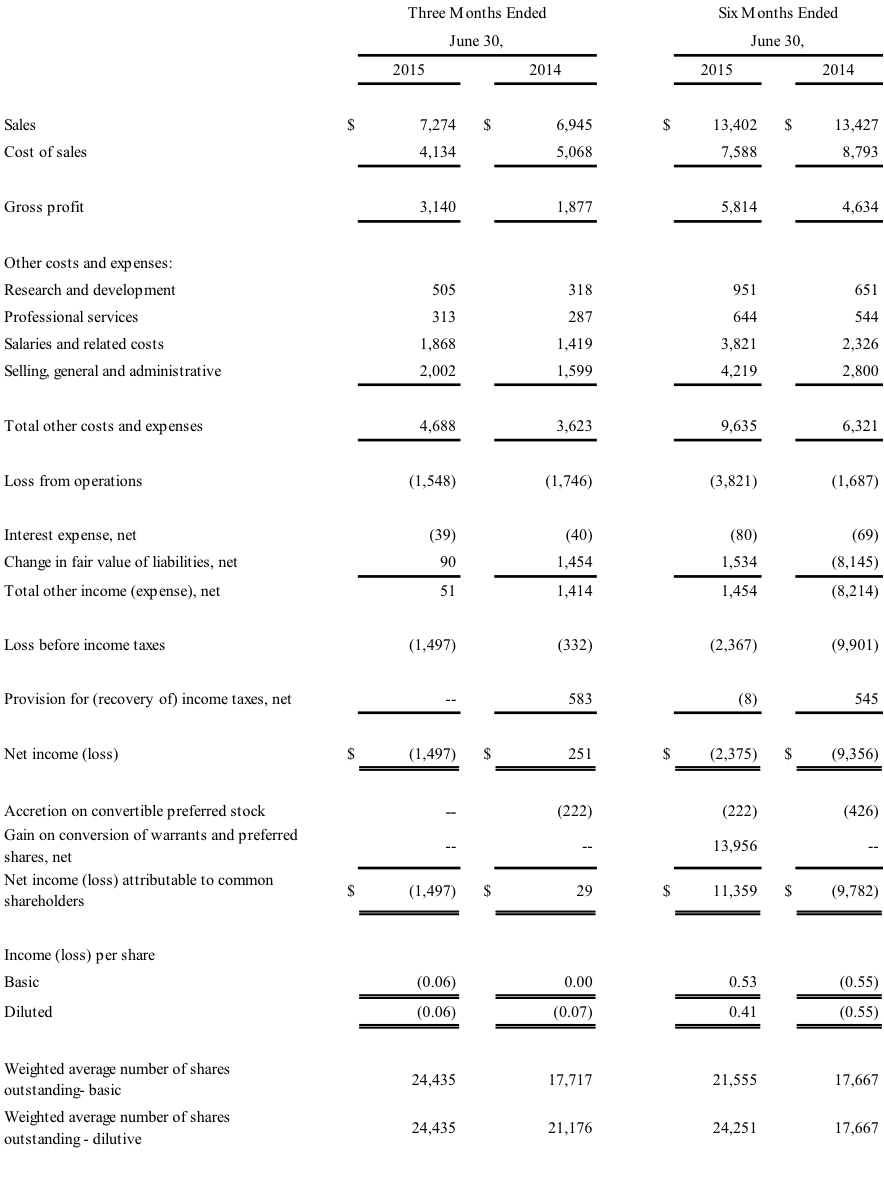
- Sales increased 4.7% year-on-year and 19% sequentially
- Gross margin expanded to 43.2%, compared with adjusted gross margin of 39.2% in 2Q14
- Formation of Medical Advisory Board to extend use of J-Plasma® to additional specialties
J-Plasma® Update
- Q2 sales of $170,000; July shipments were $237,000
- Generators in use totaled 34, up from 24 at end of Q1
- 42 Scrub Purchase Orders in H1 2015 compared to 6 in 2014
- Systems approved by 42 Hospital Value Analysis Committees (VACs); Under review by an additional 46 VACs
- Two additional Independent Delivery Networks (IDNs) signed as customers
CLEARWATER, FL — August 6, 2015 – Bovie Medical (NYSE:BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced results for the second quarter ended June 30, 2015.
Management Comments
“Second quarter revenue performance represented solid year-on-year and sequential growth in line with our strategy of expanding Bovie’s core product business, while making consistent progress on the commercialization of J-Plasma®, ” said Robert L. Gershon, Chief Executive Officer.
“This was our second consecutive quarter of six-figure sales for J-Plasma®, but longer sales cycles for hospital based products capped the sequential growth that we had expected. We are pleased to report, however, that July shipments of J-Plasma® were $237,000, a strong signal of the growing acceptance and use of this transformational product.
“There are several leading indicators that underpin our confidence in a significant pick-up in J-Plasma® sales momentum. At the end of the second quarter, there were 34 generators in use, up from 24 at the end of the prior quarter; we had 30 ordering accounts, up from 21 the end of the first quarter; and, J-Plasma has been approved by 42 Hospital Value Analysis Committees (VACs) to date and is under review at an additional 46. In the first half of 2015, we received 42 scrub purchase orders, which are used by surgeons to secure products while they are under VAC review. Scrub purchase orders are an early indication of future sales trends, and the fact that we received 42 compared to 6 for all of last year demonstrates the growing surgeon adoption of J-Plasma®. In fact, at the end of the second quarter, there were 76 surgeons using J-Plasma® compared to 41 at the end of this year’s first quarter.
“We continue to expand our R&D and business development activities to maintain our leadership and unlock potential in new markets. In the core business, we have launched two new products under the Bovie brand, which fit well in Gynecology and Dermatology specialties. The Colpo-MasterTM offers higher magnification for gynecologists, improving their ability to diagnose due to sharper visualization. Another product, FreezpointTM, is a device for dermatologic treatment of skin lesions, and enables a convenient method for freezing tissue,” Mr. Gershon noted.
Second Quarter 2015 Results
Second quarter sales were $7.3 million, up 4.7% from $6.9 million in the second quarter of 2014 on higher sales of core products. Gross margin was 43.2%, compared with a gross margin adjusted for an inventory write-down of 39.2% in the second quarter of 2014.
Operating expenses totaled $4.7 million in the second quarter, compared with $3.6 million in the second quarter of 2014. Approximately 55% of the increase was due to higher spending to accelerate the commercialization of J-Plasma and further develop Bovie’s R&D capability. The Company incurred an operating loss of $1.5 million, compared with an operating loss of $1.7 million in the second quarter of 2014.
GAAP net loss for the second quarter was $1.5 million, or $0.06 per diluted share, compared with GAAP net income of $251,000, with $29,000 attributable to common shareholders, in the second quarter of 2014. Results for the second quarter of 2014 included a non-cash gain of $1.5 million related to mark-to-market fair value of issued common stock repurchase warrants.
Recent Developments
- Bovie has established a Medical Advisory Board that will consist of surgeons who are thought-leaders in a range of different specialties and named Dr. Vipul Patel, a world-renowned urologist and leader in robotic surgery, as its first member. Ultimately, the Board is expected to have 6 to 9 members and lead acceptance of J-Plasma® in new target specialties including, but not limited to, cardiovascular, cardiothoracic and urology.
“Our ability to attract a surgeon of the caliber of Dr. Patel to our Advisory Board is further evidence of the technological advantages of J-Plasma®,” Mr. Gershon noted.
Dr. Patel added, “J-Plasma® is one of the most significant innovations in energy in more than a decade, and it has the potential to be of tremendous benefit to patients undergoing surgery for prostate cancer.”
Summary and Outlook
“We have made significant progress in the first six months of 2015 and are looking ahead to a strong second half. Our core business continues to post solid results, and we are moving forward with R&D investments that are focused on expanding the Bovie product portfolio and addressing new markets. Additionally, our second half comparisons should benefit from new OEM contracts that are scheduled to go into the production phase in the coming months.
“At the same time, the key business metrics we have quantified, including numbers of generators in use, ordering customers, VAC approvals, scrub POs and the like all point to a strong pick-up in J-Plasma® sales in the second half of this year. We have an excellent record of VAC approvals, and we recently signed agreements with two new IDNs, which gives us access to a broad base of potential surgeon users in their combined 21 member hospitals. Thus, while timing issues have extended our commercialization process by a few months, we believe that all the elements are in place for substantial improvement in J-Plasma® sales in the coming periods,” Mr. Gershon said.
Conference Call Details
The Company’s management will host a conference call on Thursday, August 6, 2015 at 5pm Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150806.html
A replay of the call will be available approximately one hour after the end of the call through November 6, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10068946.
Investor Relations Contact
MBS Value Partners
Hugh Collins
212-223-4632
investor.relations@boviemed.com
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-K and 10-Q for the year ended December 31, 2014 and the quarter ended March 31, 2015, respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
BOVIE MEDICAL CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2015 AND 2014
(UNAUDITED) (in thousands except per share data)
 (Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
(Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
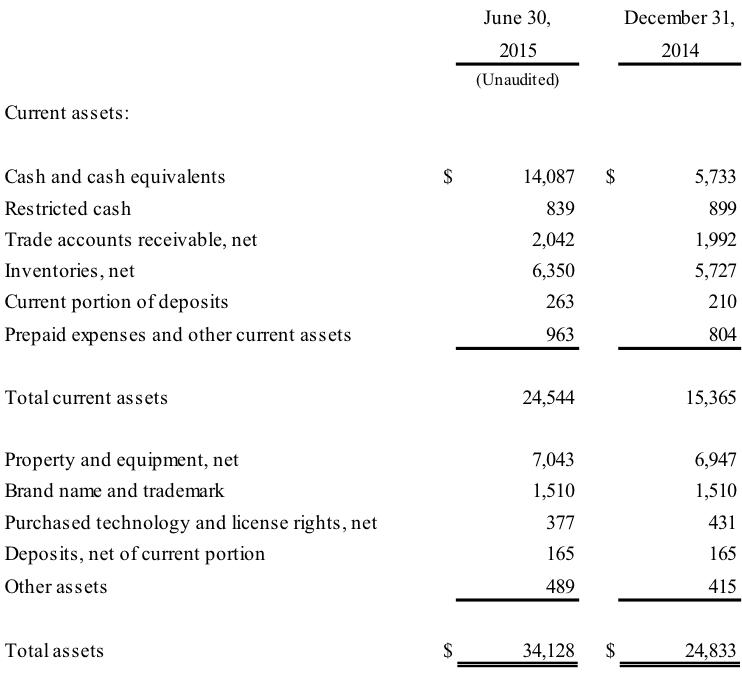
BOVIE MEDICAL CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands)
 (Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
(Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
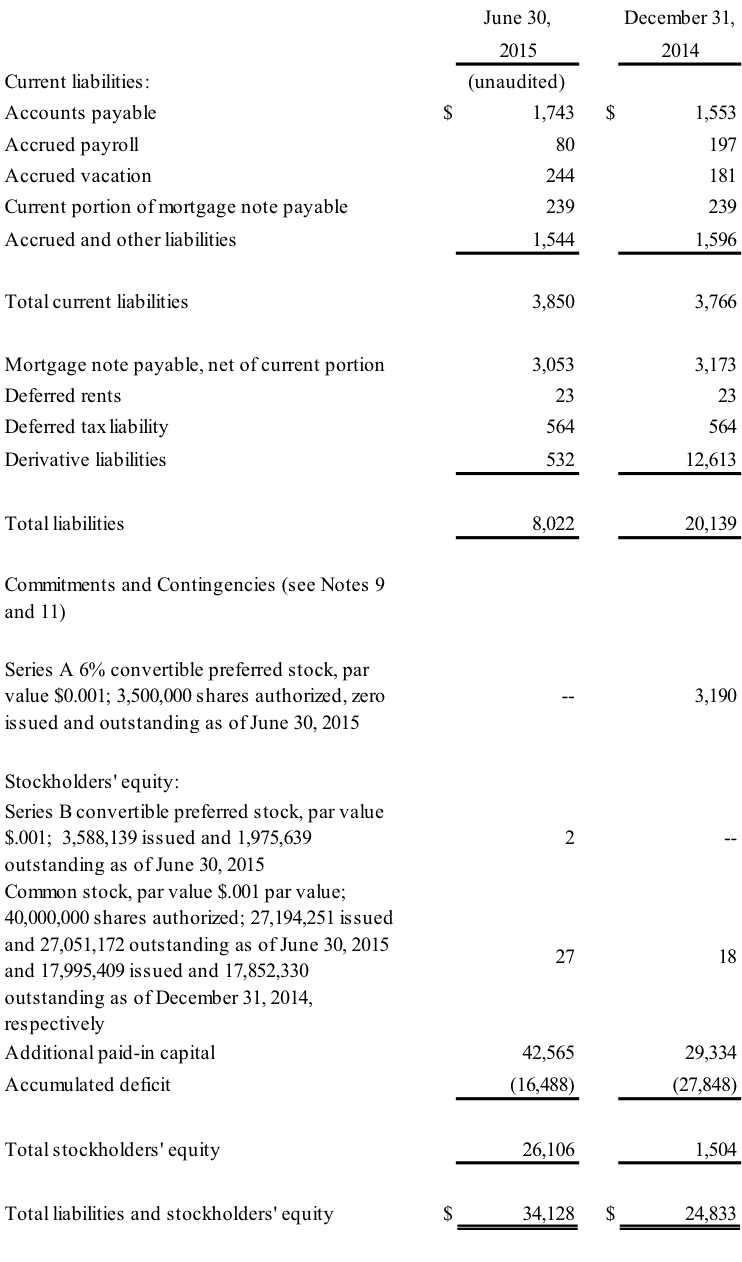
BOVIE MEDICAL CORPORATION
CONSOLIDATED BALANCE SHEETS
(CONTINUED) (in thousands)
 (Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
(Note 1) Amounts reflected in the presentation of calculations may be impacted by rounding.
CLEARWATER, FL — July 23, 2015 – Bovie Medical (BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced that it will issue its financial results for the second quarter ended June 30, 2015 on Thursday August 6, 2015 after market close.
Management will host a conference call on Thursday August 6, 2015 at 5:00pm Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150806.html
A replay of the call will be available approximately one hour after the end of the call through August 14, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10068946.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Investor Relations Contact
MBS Value Partners
Lynn Morgen and Hugh Collins
212.750.5800
investor.relations@boviemed.com
CLEARWATER, FL — June 18, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that Robert L. Gershon, Chief Executive Officer of the Company, will present at the JMP Securities Life Sciences Conference at 11.30am EST on June 24, 2015 at the St. Regis Hotel in New York.
During his presentation, Mr. Gershon will discuss 2015 targets and milestones to date for J-Plasma, Bovie’s transformational surgical product which enables surgeons to operate with greater precision on and around delicate structures with minimal thermal spread.
The presentation will be webcast at http://boviemedical.wpengine.com/financials.asp
The JMP Securities’ annual life sciences equity research conference is an institutional investor forum featuring publicly traded and privately held companies in the areas of biotechnology, specialty pharmaceuticals, medical devices, life science tools and diagnostic.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
CLEARWATER, FL — June 15, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies, and the developer of J-Plasma®, a patented new surgical product, today announced that Peter Donato, Chief Financial Officer, will leave the company on or about June 25, 2015 to take a position in his home state of Ohio.
Jay Ewers, Corporate Controller, has been appointed interim CFO effective upon Mr. Donato’s departure. Mr. Ewers has more than 30 years of senior experience and has held financial executive positions in corporations ranging from early stage to high profile public companies with global operations in the medical equipment, manufacturing and semiconductor industries. He has extensive experience with Sarbanes Oxley, inventory management, Oracle modules and financial planning & analysis. Mr. Ewers is a certified public accountant, internal auditor, and holds a BS in Accounting.
Bovie Medical will engage an executive search firm to conduct a search for a permanent CFO.
“We appreciate Peter’s contribution during the time he has been with us and wish him the best,” said Robert L. Gershon, Chief Executive Officer. “We are fortunate to have a senior executive with Jay’s experience and a supporting financial team. Jay has played a key role in managing finance-related functions at Bovie Medical.” Mr. Donato has entered into a consulting agreement to assist Mr. Ewers’ seamless transition to interim CFO until a permanent CFO is named.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-Q for the quarter ended March 31, 2015 and 10-K for the year ended December 31, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins /Jane Searle
MBS Value Partners
(212) 750-5800
investor.relations@boviemed.com
CLEARWATER, FL — May 26, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies, and the developer of J-Plasma®, a patented new surgical product, today announced that it has hired Mark Charbonneau as J-Plasma® Product Development Manager.
Prior to joining Bovie, Mark was an engineering manager for Centrix and senior manufacturing engineer for Ventripoint Diagnostics. Previously, Mark worked in senior engineering roles at companies including Cenorin, Archus Orthopedics and Salient Surgical Technologies. He brings more than 14 years of experience to Bovie and received a B.S. in engineering from the University of Massachusetts.
“This hire is part of our broader strategy to expand a highly-credentialed team to accelerate the adoption of J-Plasma,” said Robert L. Gershon, Chief Executive Officer. “Mark comes to Bovie Medical with a proven track record in new medical device roll-outs, product design and a strong technical background.”
Mr. Charbonneau’s hire was effective May 18, 2015. He reports to Mr. Shawn Roman, Director of Research and Development.
The terms of Mark’s employment with Bovie include an inducement grant of 10,000 options with a 4 year vesting period. The strike price of the inducement grant will equal the closing price as of today, May 26, 2015. The options shall vest and be exercisable in four equal annual installments beginning on the first anniversary of the effective date.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-Q for the quarter ended March 31, 2015 and 10-K for the year ended December 31, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
- Strong sales momentum achieved by J-Plasma®
- Gross margin was 43.6%
- Higher core products sales partially offset the anticipated reduction in OEM revenues
- Net proceeds of equity offering to fund future growth
J-Plasma® Update
- First quarter sales of $283,370, 35% ahead of full year 2014 sales
- 21 ordering accounts, up from 18 at the end of 2014
- Systems approved by 35 Hospital Value Analysis Committees (VACs)
CLEARWATER, Fl. – May 8, 2015- Bovie Medical (NYSE:BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced results for the first quarter ended March 31, 2015.
Management Comments
“First quarter 2015 was a period of strong execution for Bovie across key metrics,” said Robert L. Gershon, Chief Executive Officer. “We continued to post year-over-year growth in core product sales, led by electrodes and medical lighting, which partially mitigated the impact of lower OEM revenues, where several projects underway are not scheduled to go into production until later this year.
“Importantly, this was a very strong quarter for J-Plasma® sales, which exceeded full year 2014 sales by over 35%, a testament to the growing awareness and adoption of the product. At the end of the period we had 21 ordering accounts, defined as accounts that have adopted the technology as evidenced by placing re-orders of J-Plasma®. Our systems have been approved by 35 Hospital Value Analysis Committees (VACs) and are currently being evaluated by an additional 34 throughout the country, setting the stage for continued positive momentum.
“Approximately 50% of the year-on-year increase in first quarter operating expenses was directly related to the commercialization of J-Plasma® and to R&D spending to drive new product development. Based on our current portfolio, this spending level is expected to remain relatively constant throughout the remaining quarters of 2015. The net proceeds raised in our recent public offering provide us with financial flexibility and will be used to support our growth initiatives.”
First Quarter 2015 Results
First quarter sales were $6.1 million, a 5.5% decline from the $6.5 million reported in last year’s first quarter, as higher core product and J-Plasma® sales only partly offset the anticipated reduction in OEM revenues. Gross margin expanded to 43.6% from 42.5%.
Operating expenses increased by 83% year-on-year as a result of significantly higher spending levels to accelerate the commercialization of J-Plasma®, expand R&D activities, and build the infrastructure needed to support Bovie’s future growth. The operating loss was $2.3 million compared to an operating profit of $58,000 in the first quarter of 2014.
The Company had GAAP income of $12.9 million, or $0.57 per diluted share. This compares to GAAP loss of $9.8 million in last year’s first quarter. Both quarters were impacted by mark-to-market fair value of issued common stock purchase warrants. Exclusive of these non-cash gains and losses, the Company would have reported a net loss of $2.5 million vs a loss of $212,000 last year.
Recent Developments
- On March 31, 2015, the Company completed an underwritten public offering of 5,218,749 shares of its common stock, including an over-allotment option, at a price to the public of $2.50 per share. The estimated net proceeds from the offering were approximately $11.5 million. Great Point Partners subscribed to the public offering as did other well-known institutional healthcare investors.
- Upon the completion of the above-mentioned public offering, Great Point Partners, LLC agreed to convert its holdings of 3.5 million shares of the Company’s Series A 6% Convertible Preferred Stock and warrants to purchase up to 5,250,000 shares of Bovie’s common stock into 3,588,139 shares of the Company’s newly-issued Series B Convertible Preferred Stock, which is convertible into an aggregate of 7,176,278 shares of the Company’s common stock.
Commenting on these developments, Mr. Gershon said, “We took advantage of market conditions to raise the funds needed to accelerate the commercialization of our transformational J-Plasma® product and to gain the resources to pursue additional product development opportunities. We are pleased to have built a base of institutional ownership through this offering, and that we were able to reach an agreement with Great Point that will enable our future net income results to more closely track our operating progress.”
- Mr. Charles Orsatti has joined the Board as an independent director effective May 6, 2015, replacing Ian Sheffield who is stepping down as a director in connection with his departure from Great Point Partners (GPP). Mr. Orsatti comes to Bovie with more than 35 years of experience at the executive management level in public and private medical device companies, both in the United States and Europe, with an emphasis on start-up and high growth businesses. He is currently chairman and a director of the Biotronic Neuronetwork, a provider of inter-operative neurological monitoring services. Previously, Mr. Orsatti served as a board member of Angiodynamics, SRI Surgical, Ossur Americas, LTD, and dj Orthopedics, following a successful career in private equity and as CEO of Fairfield Medical Products Corporation and Coloplast, Inc.
“We are pleased to welcome Charles Orsatti to the Bovie Board. Charles’ board credentials are supported by over 20 years of direct executive management experience in the healthcare field, which will be invaluable as we scale up our business in the coming years,” Mr. Gershon said.
Summary and Outlook
“We believe that the key elements are in place for the effective execution of our growth strategy in 2015, led by full commercialization of J-Plasma® and related products. Specifically, we are targeting:
- An increase in the number of ordering accounts to 125 from the current 21
- To reach 250 surgeons using J-Plasma® compared to 41 today
- The expansion of our direct sales force to 20 from 16
- Publication of 10-15 independent white papers, including the 5 published in 2014
- An increase in the number of key opinion leaders speaking on J-Plasma® to 10-15 from 8
“At the same time, we are working toward building our core business at a strong, sustainable pace. This involves making ongoing R&D investments to reinforce our market leadership and unlock the value of our product and patent portfolio, expanding internationally, and addressing new markets,” Mr. Gershon concluded.
Conference Call Details
The Company’s management will host a conference call on Friday, May 8, 2015 at 9am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150508.html
A replay of the call will be available approximately one hour after the end of the call through May 16, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10064668.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Forms 10-K for the year ended December 31, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
|
BOVIE MEDICAL CORPORATION CONSOLIDATED STATEMENTS OF OPERATIONS |
||||
|
Three Months Ended
March 31, 2015 |
||||
|
2015
|
2014
|
|||
|
Sales
|
$ 6,128
|
$ 6,482
|
||
|
Cost of sales
|
3,454
|
3,726
|
||
|
Gross Profit
|
2,674
|
2,756
|
||
|
Other costs and expenses:
|
||||
|
Research and development
|
446
|
332
|
||
|
Professional services
|
331
|
258
|
||
|
Salaries and related costs
|
1,952
|
907
|
||
|
Selling, general, and administrative
|
2,217
|
1,201
|
||
|
Total other costs and expenses
|
4,946
|
2,698
|
||
|
Loss from operations
|
(2,272)
|
58
|
||
|
Interest and other expense, net
|
(40)
|
(28)
|
||
|
Change in fair value of liabilities, net
|
1,444
|
(9,599)
|
||
|
Total other expenses, net
|
1,404
|
(9,627)
|
||
|
Loss before income taxes
|
(868)
|
(9,569)
|
||
|
Provision for income taxes, net
|
(8)
|
(38)
|
||
|
Net (loss)
|
$ (876)
|
$ (9,607)
|
||
|
Accretion on convertible preferred
stock |
(222)
|
(204)
|
||
|
Deemed dividend on conversion of warrants and Series A preferred stock to Series B convertible preferred stock
|
13,956
|
–
|
||
|
Net Income (loss) attributable to common shareholders
|
$ 12,858
|
$ (9,811)
|
||
| Income (loss) per share: | ||||
|
Basic
|
0.69
|
(0.55)
|
||
|
Diluted
|
0.57
|
(0.55)
|
||
|
Weighted average number of shares:
|
||||
|
Basic
|
18,615
|
17,684
|
||
|
Diluted
|
20,470
|
17,684
|
||
|
BOVIE MEDICAL CORPORATION CONSOLIDATED BALANCE SHEETS (unaudited) (in thousands) |
||||
| ASSETS |
March 31, 2015
(Unaudited) |
December 31, 2014
|
||
|
Current assets:
|
||||
|
Cash and cash equivalents
|
$ 15,396
|
$ 5,733
|
||
|
Restricted cash
|
839
|
899
|
||
|
Trade accounts receivable, net
|
1,398
|
1,992
|
||
|
Inventories, net
|
6,130
|
5,727
|
||
|
Current portion of deposits
|
286
|
210
|
||
|
Prepaid expenses and other current assets
|
1,011
|
804
|
||
|
Total current assets
|
25,060
|
15,365
|
||
|
Property and equipment, net
|
7,011
|
6,947
|
||
|
Brand name and trademark
|
1,510
|
1,510
|
||
|
Purchased technology and license rights, net
|
404
|
431
|
||
|
Deposits, net of current portion
|
164
|
165
|
||
|
Other assets
|
439
|
415
|
||
|
Total assets
|
$34,588
|
$24,833
|
||
|
BOVIE MEDICAL CORPORATION CONSOLIDATED BALANCE SHEETS |
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||
|
March 31, 2015 (unaudited)
|
December 31, 2014
|
|||
|
Current liabilities:
|
||||
|
Accounts payable
|
$1,876
|
$1,553
|
||
|
Accrued payroll
|
118
|
197
|
||
|
Accrued vacation
|
209
|
181
|
||
|
Current portion of mortgage note payable
|
239
|
239
|
||
|
Deferred revenue
|
5
|
—
|
||
|
Accrued and other liabilities
|
1,629
|
1,596
|
||
|
Total current liabilities
|
4,076
|
3,766
|
||
|
|
||||
|
Mortgage note payable, net of current portion
|
3,113
|
3,173
|
||
|
Deferred rents
|
22
|
23
|
||
|
Deferred tax liability
|
563
|
564
|
||
|
Derivative liabilities
|
622
|
12,613
|
||
|
Total liabilities
|
8,396
|
20,139
|
||
|
Commitments and Contingencies
|
||||
|
Series A 6% convertible preferred stock, par value $0.001; 3,500,000 shares authorized, zero issued and outstanding as of March 31, 2015
|
—
|
3,190
|
||
|
Stockholders’ equity:
|
||||
|
Series B convertible preferred stock, par value $.001; 10,000,000 authorized, 3,588,139 issued and outstanding as of March 31, 2015
|
4
|
—
|
||
|
Common stock, par value $.001 par value; 40,000,000 shares authorized; 23,335,894 issued and 23,192,815 outstanding as of March 31, 2015 and 17,995,409 issued and 17,852,330 outstanding as of December 31, 2014, respectively
|
23
|
18
|
||
|
Additional paid-in capital
|
41,155
|
29,334
|
||
|
Accumulated deficit
|
(14,990)
|
(27,848)
|
||
|
Total stockholders’ equity
|
26,192
|
1,504
|
||
|
Total liabilities and stockholders’ equity
|
$34,588
|
$24,833
|
||
|
BOVIE MEDICAL CORPORATION |
||||
|
Three Months Ended
March 31, 2015 |
||||
| (Amounts in ‘000’s except earnings per share) |
2015
|
2014
|
||
|
Net income/(loss) GAAP basis
|
$ (876)
|
$ (9,608)
|
||
|
Accretion on convertible preferred stock
|
(222)
|
(204)
|
||
|
Deemed dividend on conversion of warrants and Series A convertible preferred to Series B convertible preferred stock
|
13,956
|
–
|
||
|
Net income/(loss) attributable to common shareholders
|
$12,858
|
$ (9,811)
|
||
|
Net income/(loss) per share – basic (GAAP basis)
|
$ 0.69
|
$ (0.55)
|
||
|
Other non-GAAP adjustments:
|
||||
|
(Gain)/loss on change in fair value of derivative liabilities
|
$ (1,444)
|
$ 9,599
|
||
|
Deemed dividend on conversion of warrants and Series A convertible preferred to Series B convertible preferred stock
|
(13,956)
|
–
|
||
|
Adjusted non-GAAP net income/(loss)
|
(2,542)
|
(212)
|
||
|
Income/(loss) per share – basic on: (Note 1)
|
||||
|
(Gain)/loss on change in fair value of derivative liabilities
|
$(0.08)
|
$0.54
|
||
|
Gain on conversion of warrants and Series A convertible preferred to Series B convertible preferred stock
|
(0.75)
|
–
|
||
|
Adjusted non-GAAP net (loss) per share -basic
|
(0.14)
|
(0.01)
|
||
|
|
||||
|
Weighted average number of shares outstanding – basic
|
18,615
|
17,684
|
||
|
(Note 1) Amounts reflected in the presentation of EPS calculations may be impacted by rounding
|
||||
Investor Relations Contact
MBS Value Partners
Lynn Morgen and Hugh Collins
212.750.5800
investor.relations@boviemed.com
CLEARWATER, FL — April 24, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced that it will issue its financial results for the first quarter ended March 31, 2015 on Friday May 8, before market open.
Management will host a conference call on Friday, May 8, 2015 at 9:00am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150508.html
A replay of the call will be available approximately one hour after the end of the call through May 16, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10064668.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
CLEARWATER, FL — March 31, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it has closed the sale of an additional 418,749 shares of its common stock pursuant to the over-allotment option of the underwriter in connection with its recent public offering of common stock. After closing of the over-allotment, the total number of shares sold by Bovie in the offering is 5,218,749. The total net proceeds of the offering, after deducting underwriting discounts, commissions and estimated offering expenses, are approximately $11.6 million.
Craig-Hallum Capital Group LLC acted as sole managing underwriter for the offering.
The shares are being offered pursuant to an effective shelf registration statement on Form S-3 that was previously filed with the Securities and Exchange Commission (SEC). The securities may be offered only by means of a prospectus. The prospectus and prospectus supplement related to the offering have been filed with the SEC and are available on the SEC’s website located at http://www.sec.gov and may also be obtained from Craig-Hallum Capital Group LLC, 222 South Ninth Street, Suite 350, Minneapolis, MN 55402, telephone 612-334-6300, email: prospectus@chlm.com.
This press release shall not constitute an offer to sell, or the solicitation of an offer to buy, nor may there be any sale of these securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K and 10-Qs for the year ended December 31, 2014 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
CLEARWATER, FL — March 17, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, announced today that it has closed its previously-announced registered underwritten public offering of 4,800,000 shares of its common stock at a price to the public of $2.50 per share. Bovie estimates net proceeds from the offering to be approximately $10.6 million, after deducting underwriting discounts and commissions and estimated offering expenses. Bovie intends to use the proceeds from the offering for working capital and general corporate purposes.
Craig-Hallum Capital Group LLC acted as sole managing underwriter for the offering.
The shares are being offered pursuant to an effective shelf registration statement on Form S-3 that was previously filed with the Securities and Exchange Commission (SEC). The securities may be offered only by means of a prospectus. The prospectus and prospectus supplement related to the offering have been filed with the SEC and are available on the SEC’s website located at http://www.sec.gov and may also be obtained from Craig-Hallum Capital Group LLC, 222 South Ninth Street, Suite 350, Minneapolis, MN 55402, telephone 612-334-6300, email: prospectus@chlm.com.
This press release shall not constitute an offer to sell, or the solicitation of an offer to buy, nor may there be any sale of these securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K and 10-Qs for the year ended December 31, 2014 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
CLEARWATER, FL — March 12, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced that it has priced an underwritten public offering of 4,800,000 shares of its common stock at a price to the public of $2.50 per share. In addition, Bovie has granted the underwriter a 30-day option to purchase up to 720,000 additional shares of common stock to cover over-allotments, if any. The offering is expected to close on or about March 17, 2015, subject to satisfaction of customary closing conditions.
The total gross proceeds of the offering are expected to be approximately $12.0 million. After deducting the underwriter’s discount and other estimated offering expenses payable by Bovie, the net proceeds to the Company are expected to be approximately $10.6 million. These amounts assume no exercise of the underwriter’s over-allotment option.
Craig-Hallum Capital Group LLC acted as sole managing underwriter for the offering.
The shares are being offered pursuant to an effective shelf registration statement on Form S-3 that was previously filed with the Securities and Exchange Commission (SEC). The securities may be offered only by means of a prospectus. The prospectus and a preliminary prospectus supplement related to the offering have been filed with the SEC, and a final prospectus supplement related to the offering will be filed with the SEC, and are available on the SEC’s website located at http://www.sec.gov and may also be obtained from Craig-Hallum Capital Group LLC, 222 South Ninth Street, Suite 350, Minneapolis, MN 55402, telephone 612-334-6300, email: prospectus@chlm.com.
This press release shall not constitute an offer to sell, or the solicitation of an offer to buy, nor may there be any sale of these securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K and 10-Qs for the year ended December 31, 2014 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
CLEARWATER, FL — March 11, 2015 – Bovie Medical Corporation (“Bovie” or the “Company”) (NYSE: BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced a proposed registered underwritten public offering of its common stock, subject to market and other conditions. In addition, the Company intends to grant the underwriter an option to purchase additional shares equal to up to 15% of the aggregate number of shares to be sold in the offering.
Craig-Hallum Capital Group LLC is acting as sole underwriter for the offering.
Bovie intends to use the net proceeds from the offering, if completed, for working capital and general corporate purposes.
The shares are being offered pursuant to an effective shelf registration statement on Form S-3 that was previously filed with the Securities and Exchange Commission (SEC). The securities may be offered only by means of a prospectus. The prospectus and a preliminary prospectus supplement related to the offering have been filed with the SEC and are available on the SEC’s website located at http://www.sec.gov and may also be obtained from Craig-Hallum Capital Group, 222 South Ninth Street, Suite 350, Minneapolis, MN 55402, telephone 612-334-6300, email: prospectus@chlm.com.
This press release shall not constitute an offer to sell, or the solicitation of an offer to buy, nor may there be any sale of these securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K and 10-Qs for the year ended December 31, 2014 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
Fourth Quarter Highlights
- Sales increased 22% year-on-year to $7.5 million
- Expanded R&D activities
- Bovie® Ultimate™ Generator receives 510(k) approval
J-Plasma® Update
- Q4 sales of $73,000; Full year sales of $209,000;
- 18 ordering accounts at year-end, up from 14 at the end of Q3;
- Systems Being Evaluated at 34 Hospital Value Analysis Committees (VACs)
- Increased direct sales force to 16
February 27, 2015 07:30 AM Eastern Standard Time
CLEARWATER, Fla.–(BUSINESS WIRE)–Bovie Medical (BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced results for the fourth quarter and full year ended December 31, 2014.
Management Comments
“We are pleased to report that we ended 2014 with positive momentum and with the key elements in place for excellent execution in 2015,” said Robert L. Gershon, Chief Executive Officer. “We succeeded in posting another quarter of double-digit revenue growth in core products, led by sales of generators, electrodes and lighting, and have expanded our R&D activities to leverage the value of the Bovie brand, product pipeline and patent portfolio.
“J-Plasma® sales for the fourth quarter increased almost seven times from the comparable year-ago period, bringing full year 2014 sales to $209,000, or approximately five times the $42,000 reported in 2013. We progressively added experienced health care sales professionals to create a direct sales force in 2014, supplemented with a dedicated group of 20 independent manufacturers’ representatives. At the end of 2014, we had 18 ordering accounts, defined as accounts that have re-ordered the J-Plasma®, product, and our systems were under evaluation at 34 Hospital Value Analysis Committees (VACs), up from 20 at the end of this year’s third quarter. This increase reflects the expanding number of institutions and surgeons that have recognized the exceptional benefits of J-Plasma® in eliminating or significantly lowering the risk of collateral thermal injury to healthy soft tissue surrounding an operative site.
“The investments we made in the roll-out of J-Plasma® and in R&D spending resulted in higher operating expenses in the fourth quarter and full year, which we are confident will begin to yield solid returns in 2015,” Mr. Gershon noted. “In the first two months of 2015, J-Plasma® sales have already exceeded those of the fourth quarter, and we are confident that they will accelerate as the year progresses.”
Fourth Quarter Results
Fourth quarter sales were $7.5 million, a 21.8% increase from $6.1 million in the fourth quarter of 2013, reflecting strong demand for distributed and branded products.
Gross profit was $2.4 million compared with $2.6 million in the fourth quarter of 2013, while gross profit margin was 32.7%, compared to 41.9%. Adjusted gross margin, which excludes the impact of inventory write-downs, was 38.7% in the fourth quarter of 2014.
Operating loss for the quarter was $1.8 million, compared with $875,000 in the fourth quarter of 2013, reflecting increased spending on J-Plasma® commercialization, which accounted for approximately 67% of the year-over-year increase. Additionally, R&D spending increased 23.7%, as the Company moved ahead with plans to optimize its existing product and patent portfolio.
The Company recorded a non-cash gain of $2.5 million related to the mark-to-market accounting for the fair value of issued common stock purchase warrants, compared with a non-cash loss of $859,000 in the fourth quarter of 2013. In addition, the Company recorded a non-cash charge of $6.0 million on the write-down of a deferred tax asset.
Net loss attributable to common shareholders for the period was $5.5 million, or $0.39 per diluted share, compared with a loss of $5.1 million, or $0.29 per diluted share, in the fourth quarter of 2013.
Full Year 2014 Results
For full year 2014, total sales were $27.7 million, up 17% from $23.7 million in 2013. Gross profit was $9.0 million, compared to 2013’s gross profit of $9.2 million. Adjusted gross margin, which excludes costs related to excess and obsolete inventory and other adjustments, was 40.7%, up from 38.9% in 2013.
Operating loss in 2014 was $5.8 million, compared with $3.7 million in 2013. The Company’s full year 2014 operating costs included $2.6 million related to the production and marketing of J-Plasma®, up from $1.3 million in 2013.
The Company recorded a non-cash loss of $7.3 million related to the mark-to-market accounting for the fair value of issued common stock purchase warrants, compared with a non-cash loss of $842,000 in 2013.
The Company reported a net loss of $18.2 million, or $1.03 per diluted share, compared with $7.0 million, or $0.40 per diluted share, in 2013. Non-GAAP adjusted net loss, which excludes the non-cash loss related to warrants and other adjustments, was $5.3 million or $0.30 per basic share, compared with $3.8 million or $0.21 per basic share, in 2013.
Summary and Outlook
“At the beginning of 2014, we stated that our main objective was to build the appropriate foundation for substantial and sustainable long-term growth and to report milestones as they occurred over the course of the year. We are pleased to have accomplished this objective, and to have improved both the performance and growth prospects of our core business, while we moved ahead with the commercialization of J-Plasma®,” Mr. Gershon said.
Highlights of 2014 included the following:
- Five independent white papers on J-Plasma® were published in 2014, detailing the benefits of J-Plasma® when used in procedures within the Company’s initial target markets, namely gynecology, dermatology and plastic surgery.
- In 2014, the Company completed 10 surgeon training courses in six cities across the U.S.
- The Company’s direct sales force grew from zero at the end of 2013 to 16 by the end of 2014 and is comprised of a group very experienced sales professionals who have been attracted to Bovie by the attributes of its J-Plasma® product.
- Bovie hired a Director of Research and Development in line with its strategy to develop its existing R&D pipeline and leverage its in-house design capabilities and manufacturing facility.
- The Company launched new products: IDS 310, Derm101 and 102 in 2014, and in December received FDA 510(k) for the Bovie® Ultimate™, a product that combines J-Plasma® technology with the highest wattage operating room electrosurgical generator and is expected to be launched in the second quarter of 2015.
- The next generation handle for the J-Plasma® product, the Pistol Grip was launched in October.
- In early January 2015, the Company received CE approval for all J-Plasma® disposable hand pieces and the Bovie® Ultimate™ generator, enabling it to sell these devices in the 31 of the European Economic Area.
“In summary, 2014 was an investment year for Bovie, as we moved forward with initiatives that have strengthened our core business, while we have remained keenly focused on accelerating the adoption of J-Plasma® nationwide. With a trained and motivated sales force in place, a surgeon-user base that has experienced superior outcomes, and a product with the potential to be a transformational technology across a broad range of surgical specialties, we are entering 2015 with positive momentum and ambitious growth objectives,” Mr. Gershon concluded.
Recent hires
Bovie Medical made several important hires in recent months including:
- Ms. Stephanie Van Skike was appointed as Western US Regional Sales Manager
- Mr. Brian Kunst was appointed Vice President of Clinical and Regulatory Affairs
- Mr. Brad Boklage was appointed as Senior Surgical Device Specialist
- Mr. Benjamin Kirsch was appointed as Senior Surgical Specialist
In addition, Bovie Medical is pleased to announce the hiring of Mr. Christopher R. Corp as Director, National Accounts, effective February 2, 2015. Mr. Corp has more than 15 years of experience in healthcare, with companies including Kimberly-Clark and Medical Action Industries. He will report to Jack McCarthy, Chief Commercialization Officer. Mr. Corp has been awarded non-qualified stock options to purchase 20,000 shares of common stock, exercisable at the closing price as of today, February 27, 2015. The options shall vest and be exercisable in four equal installments beginning on the first anniversary of the effective date.
Conference Call
The Company’s management will host a conference call on Friday, February 27, 2015 at 9:00am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150227.html
A replay of the call will be available approximately one hour after the end of the call through March 7, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10060385.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Use of Non-GAAP Financial Measures
In this press announcement, management has disclosed financial measurements that present financial information not in accordance with Generally Accepted Accounting Principles (GAAP). These measurements are not a substitute for GAAP measurements, although company management uses these measurements as aids in monitoring the company’s ongoing financial performance from quarter to quarter and year to year on a regular basis and for benchmarking against other medical technology companies. Adjusted non-GAAP gross margin, adjusted non-GAAP income from operations, adjusted non-GAAP net income and adjusted non-GAAP income per diluted share measure the gross margin, income from operations, net income and income per diluted share of the company excluding unusual items. Management uses and presents these measures because management believes that such adjustments facilitate an understanding of the financial impact of unusual items on the company’s short- and long-term financial trends. Management also uses adjusted non-GAAP items to forecast and to evaluate the operational performance of the company, as well as to compare results of current periods to prior periods on a consistent basis.
Non-GAAP financial measures used by the company may be calculated differently from, and therefore may not be comparable to, similarly-titled measures used by other companies. Investors should consider non-GAAP measures in addition to, and not as a substitute for, or superior to, financial performance measures prepared in accordance with GAAP.
Please refer to the attached reconciliation between GAAP and non-GAAP financial measures.
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K/A and 10-Q for the year ended December 31, 2013 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014, respectively. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
|
BOVIE MEDICAL CORPORATION CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS |
||||
|
Three Months Ended
December 31, |
Years Ended
December 31, |
|||
|
2014
|
2013
|
2014
|
2013
|
|
|
Sales
|
$ 7,467
|
$ 6,128
|
$ 27,681
|
$ 23,660
|
|
Cost of sales
|
5,026
|
3,560
|
18,689
|
14,462
|
|
Gross Profit
|
2,441
|
2,568
|
8,992
|
9,198
|
|
Other costs and expenses:
|
||||
|
Research and development
|
398
|
321
|
1,416
|
1,260
|
|
Professional services
|
330
|
488
|
1,016
|
1,835
|
|
Salaries and related costs
|
1,743
|
1,423
|
5,723
|
3,992
|
|
Selling, general, and administrative
|
1,762
|
1,212
|
6,686
|
5,777
|
|
Legal award
|
–
|
–
|
–
|
–
|
|
Total other costs and expenses
|
4,233
|
3,443
|
14,841
|
12,864
|
|
Loss from operations
|
(1,792)
|
(875)
|
(5,849)
|
(3,666)
|
|
Interest and other expense, net
|
(40)
|
(1,274)
|
(151)
|
(1,444)
|
|
Change in fair value of liabilities, net
|
2,535
|
(859)
|
(7,285)
|
(842)
|
|
Income (loss) before income taxes
|
703
|
(3,009)
|
(13,285)
|
(5,952)
|
|
Benefit (provision) for deferred income taxes, net
|
(5,893)
|
539
|
(3,997)
|
1,613
|
|
Net (loss)
|
$ (5,190)
|
$ (2,470)
|
$ (17,282)
|
$ (4,339)
|
|
Accretion on convertible preferred
stock |
(264)
|
(39)
|
(932)
|
(39)
|
|
Deemed dividend on beneficial conversion feature
|
–
|
(2,616)
|
–
|
(2,616)
|
|
Net loss attributable to common shareholders
|
$ (5,454)
|
$ (5,125)
|
$ (18,214)
|
$ (6,994)
|
| Loss per share | ||||
|
Basic
|
(0.31)
|
(0.29)
|
(1.03)
|
(0.40)
|
|
Diluted
|
(0.39)
|
(0.29)
|
(1.03)
|
(0.40)
|
|
Weighted average number of shares outstanding- basic
|
17,840
|
17,670
|
17,756
|
17,670
|
|
Weighted average number of shares outstanding – dilutive
|
20,366
|
17,670
|
17,756
|
17,670
|
|
BOVIE MEDICAL CORPORATION BALANCE SHEET DECEMBER 31, 2014 AND DECEMBER 31, 2013 (unaudited) (in thousands) |
||||
| ASSETS |
2014
|
2013
|
||
|
Current assets:
|
||||
|
Cash and cash equivalents
|
$ 5,733
|
$ 7,924
|
||
|
Restricted cash
|
899
|
–
|
||
|
Trade accounts receivable, net
|
1,992
|
1,990
|
||
|
Inventories, net
|
5,727
|
8,415
|
||
|
Current portion of deposits
|
210
|
948
|
||
|
Prepaid expenses and other current assets
|
804
|
545
|
||
|
Total current assets
|
15,365
|
19,822
|
||
|
Property and equipment, net
|
6,947
|
7,063
|
||
|
Brand name and trademark
|
1,510
|
1,510
|
||
|
Purchased technology and license rights, net
|
431
|
575
|
||
|
Deferred income tax asset, net
|
–
|
3,412
|
||
|
Deposits, net of current portion
|
165
|
120
|
||
|
Other assets
|
415
|
674
|
||
|
Total assets
|
$ 24,833
|
$ 33,176
|
||
|
BOVIE MEDICAL CORPORATION BALANCE SHEET DECEMBER 31, 2014 AND DECEMBER 31, 2014 |
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||
| LIABILITIES |
2014
|
2013
|
||
|
Current liabilities:
|
||||
|
Accounts payable
|
$1,553
|
$1,060
|
||
|
Accrued payroll
|
197
|
172
|
||
|
Accrued vacation
|
181
|
200
|
||
|
Current portion of bonds payable
|
–
|
72
|
||
|
Current portion of mortgage note payable
|
239
|
–
|
||
|
Current portion of settlement
|
–
|
541
|
||
|
Accrued and other liabilities
|
1,596
|
867
|
||
|
Total current liabilities
|
3,766
|
2,912
|
||
|
|
||||
|
Mortgage note payable, net of current portion
|
3,173
|
–
|
||
|
Bonds payable, net of current portion
|
–
|
3,185
|
||
|
Deferred rents
|
23
|
–
|
||
|
Deferred tax liability
|
564
|
|||
|
Derivative liabilities
|
12,613
|
5,749
|
||
|
Total liabilities
|
20,139
|
11,846
|
||
|
Commitments and Contingencies
|
||||
|
Series A 6% convertible preferred stock, par value $0.001; 3,500,000 shares authorized and issued; preference in liquidation – $7,442,260
|
3,190
|
2,259
|
||
|
STOCKHOLDERS’ EQUITY:
|
||||
|
Preferred stock, par value $.001; 10,000,000 shares authorize
|
–
|
–
|
||
|
Common stock, par value $.001 par value; 40,000,000 shares authorized; 17,995,409 issued and 17,852,330 outstanding on December 31, 2014 and 17,826,336 issued and 17,683,257 outstanding on December 31, 2013, respectively
|
18
|
18
|
||
|
Additional paid-in capital
|
29,334
|
28,687
|
||
|
Deficit
|
(27,848)
|
(9,634)
|
||
|
Total stockholders’ equity
|
1,504
|
19,071
|
||
|
Total liabilities and stockholders’ equity
|
$24,833
|
$33,176
|
||
|
BOVIE MEDICAL CORPORATION |
||||
| (Amounts in ‘000’s except earnings per share) |
Three Months Ended
December 31, |
Years Ended
December 31, |
||
|
2014
|
2013
|
2014
|
2013
|
|
|
Net Income/(loss) GAAP Basis
|
$ (5,190)
|
$ (2,470)
|
$ (17,282)
|
$ (4,339)
|
|
Accretion on convertible preferred stock
|
(264)
|
(39)
|
(932)
|
(39)
|
|
Deemed dividend on beneficial conversion feature
|
–
|
(2,616)
|
–
|
(2,616)
|
|
Net income/(loss) attributable to common shareholders
|
$ (5,454)
|
$ (5,125)
|
$ (18,214)
|
$ (6,994)
|
|
Net income/(loss) per share – basic (GAAP basis)
|
$ (0.31)
|
$ (0.29)
|
$ (1.03)
|
$ (0.40)
|
|
Other non-GAAP adjustments:
|
||||
|
(Gain)/loss on change in fair value of derivative liabilities
|
$ (2,535)
|
$ 859
|
$ 7,285
|
$ 842
|
|
Increase in inventory E&O reserve and other inventory adjustments
|
450
|
–
|
2,202
|
–
|
|
CFO transition costs
|
–
|
–
|
340
|
–
|
|
A/R write off and other administrative charges
|
–
|
7
|
218
|
7
|
|
Accretion/bcf on convertible preferred stock
|
264
|
2,655
|
932
|
2,655
|
|
Legal award
|
–
|
599
|
–
|
1,640
|
|
Valuation allowance on deferred tax asset
|
5,970
|
–
|
5,970
|
–
|
|
Tax impact on non-GAAP adjustments (ETR 37%)
(Note 2) |
674
|
(1,524)
|
(4,061)
|
(1,903)
|
|
Adjusted non-GAAP net income/(loss)
(Note 3) |
$(631)
|
$(2,529)
|
$(5,328)
|
$(3,753)
|
|
Income/(loss) per share – basic on:
(Note 1) |
||||
|
(Gain)/loss on change in fair value of derivative liabilities
|
$(0.14)
|
$0.05
|
$ 0.41
|
$0.05
|
|
Increase in inventory E&O reserve and other inventory adjustments
|
0.03
|
–
|
0.12
|
–
|
|
CFO transition costs
|
–
|
–
|
0.02
|
–
|
|
A/R write off and other administrative charges
|
–
|
–
|
0.01
|
–
|
|
Accretion/bcf on convertible preferred stock
|
0.01
|
0.15
|
0.05
|
0.15
|
|
Legal award
|
–
|
0.03
|
–
|
0.09
|
|
Valuation allowance on deferred tax asset
|
0.33
|
–
|
0.34
|
–
|
|
Tax impact on non-GAAP adjustments
|
0.04
|
(0.09)
|
(0.22)
|
(0.10)
|
|
Adjusted non-GAAP net (loss) per share -basic
|
$(0.04)
|
$(0.14)
|
$(0.30)
|
$(0.21)
|
|
Weighted average number of shares outstanding – basic
|
17,840
|
17,670
|
17,756
|
17,670
|
|
(Note 1) Amounts reflected in the presentation of EPS calculations may be impacted by rounding
|
||||
|
(Note 2)Includes both the statutory Federal tax rate of 34% and State tax rate of 3%
|
||||
|
(Note 3)To remain consistent with prior period reporting, management is including the Q4-2014 non-GAAP reconciliation. However, due to the magnitude and timing of the valuation allowance on the deferred tax asset, management believes that this comparison shows the company’s results more favorably and therefore recommends the use of the GAAP results for Q4 comparative purposes. On a full-year basis the non-GAAP results more accurately reflects how management analyses performance of the business.
|
||||
Investor Relations Contact
MBS Value Partners
Investor Relations:
Hugh Collins, (212) 223-4632
investor.relations@boviemed.com
or
Media:
Helaina Hovitz, (212) 661-2232
helaina.hovitz@mbsvalue.com
CLEARWATER, FL – February 9,, 2015 – Bovie Medical (BVX), a maker of medical devices and supplies and the developer of J-Plasma®, a patented new surgical product, today announced that it will issue its financial results for the fourth quarter and full year ended December 31, 2014 on Friday February 27, 2015 before market open.
Management will host a conference call on Friday, February 27, 2015 at 9:00am Eastern Time to discuss latest corporate developments. Following management’s formal remarks, there will be a question and answer session.
To listen to the call by phone, interested parties within the U.S. should call 1-888-349-0106. International callers should call 1-412-902-0131. All parties should ask for the Bovie Medical Corporation call. The conference call will also be available through a live webcast at Bovie Medical Corporation’s website or at http://services.choruscall.com/links/bvx150227.html
A replay of the call will be available approximately one hour after the end of the call through March 7, 2015. The replay can be accessed via Bovie Medical Corporation’s website or by dialing 1-877-344-7529 for U.S. callers or 1-412-317-0088 for International callers and using the replay access code 10060385.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal invasiveness and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com
of occult endometriosis sufferers
CLEARWATER, FL – January 12, 2015 – Bovie Medical Corporation (NYSE:BVX), a maker of medical devices and supplies, and the developer of J-Plasma®, a patented new surgical product, today provided an update on recent developments for J-Plasma®.
The Company is pleased to announce that all J-Plasma® disposable hand pieces as well as the Bovie® Ultimate™ generator have received CE Mark approval for compliance with European Union safety, health and environmental protection requirements. As a result, the Company will be able to sell these devices in the 31 countries of the European Economic Area.
Also, the Company announced the December publication of a new white paper on J-Plasma®, authored by Dr. Craig E. McCoy of Women’s Wellness Center in Columbia, Missouri. The White Paper addresses the use of J-Plasma® in targeting the chronic pelvic pain of occult endometriosis sufferers compared with existing options including apparent lesion removal and the complete removal of the peritoneum. The white paper highlighted benefits of using J-Plasma®, including minimized lateral and depth of thermal spread, reduced healing times and reduced risk to vital structures such as the fallopian tubes and ovaries.
The white paper is available on Bovie Medical’s website, www.boviemedical.com
In addition, the Company today announced the appointment of Ms. Stephanie Van Skike as Western US Regional Sales Manager, effective December 31, 2014. Ms. Van Skike has more than 20 years of experience in sales. Most recently, she held the position of Southwest Regional Sales Manager for TEI Biosciences Inc. Prior to that, she held positions at companies including LifeCell Corp. and Novartis Pharmaceuticals AG. Ms. Van Skike holds an MBA from the University of Phoenix, as well as a B.A. from the California State University. She will report to Todd Hornsby, Vice President of Sales.
Ms. Van Skike has been awarded non-qualified stock options to purchase 20,000 shares of common stock, exercisable at the closing price as of today, January 12, 2015. The options shall vest and be exercisable in four equal annual installments beginning on the first anniversary of the effective date.
“I am pleased to announce these important new developments for J-Plasma®, where we continue to make progress with commercialization. The CE Mark approval for the J-Plasma® hand pieces and the Bovie® Ultimate™ generator gives us access to important European markets, adding a new audience of potential users for our plasma technology, and will help speed registration in other foreign markets,” said Robert L. Gershon, chief executive officer of Bovie Medical. “The appointment of Stephanie Van Skike demonstrates our ability to attract world-class personnel for our sales team, while the latest white paper, the fifth published in 2014, is further independent verification of J-Plasma®’ s competitive advantages.”
“We look forward to keeping the market up-to-date as we continue the commercialization of J-Plasma® throughout 2015,” Mr. Gershon concluded.
About Bovie Medical Corporation
Bovie Medical Corporation is a leading maker of medical devices and supplies, as well as the developer of J-Plasma®, a patented new plasma-based surgical product for cutting and coagulation. J-Plasma® utilizes a helium ionization process to produce a stable, focused beam of ionized gas that provides surgeons with greater precision, minimal thermal spread and an absence of conductive currents through the patient during surgery. Bovie Medical Corporation is also a leader in the manufacture of a range of electrosurgical products and technologies, marketed through both private labels and the Company’s own well-respected brands (Bovie®, Aaron®, IDS™ and ICON™) to distributors worldwide. The Company also leverages its expertise through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company’s current and new products, please refer to the Investor Relations section of Bovie Medical Corporation’s website www.boviemed.com
Cautionary Statement on Forward-Looking Statements
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Reports on Forms 10-K/A and 10-Qs for the year ended December 31, 2013 and the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact
Hugh Collins
MBS Value Partners
(212) 223-4632
investor.relations@boviemed.com

