APYX MEDICAL CORPORATION REPORTS FIRST QUARTER 2022 FINANCIAL RESULTS AND UPDATES FULL YEAR 2022 FINANCIAL OUTLOOK
Advanced Energy sales increased 41% year-over-year in Q1
CLEARWATER, FL — May 12, 2022 – Apyx® Medical Corporation (NASDAQ:APYX) (the “Company”), a maker of medical devices and supplies and the developer of Helium Plasma Technology, marketed and sold as Renuvion® and J-Plasma® in surgical markets, today reported financial results for its first quarter ended March 31, 2022, and updated financial expectations for the full year ending December 31, 2022.
First Quarter 2022 Financial Summary:
- Total revenue of $12.5 million, up 45% year-over-year.
- Advanced Energy revenue of $10.8 million, up 41% year-over-year.
- OEM revenue of $1.7 million, up 72% year-over-year.
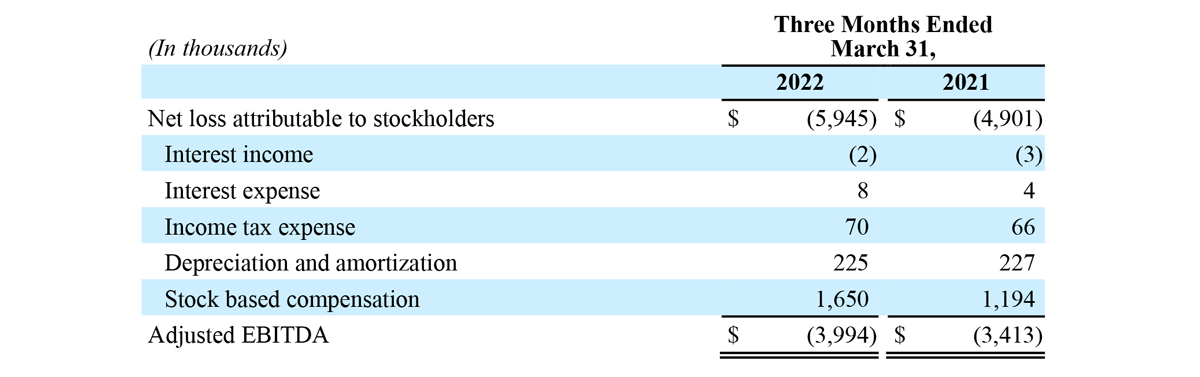
- Net loss attributable to stockholders of $5.9 million, compared to $4.9 million for the first quarter of 2021.
- Adjusted EBITDA loss of $4.0 million, compared to adjusted EBITDA loss of $3.4 million for the first quarter of 2021.
First Quarter 2022 Operating Summary:
- On February 17, 2022, the Company announced the publication of a peer-reviewed article in the journal, Lasers in Surgery and Medicine, the official journal of the American Society for Laser Medicine & Surgery, featuring the results of its U.S. IDE clinical study evaluating the Renuvion Dermal Handpiece using Apyx’s Helium Plasma Technology for dermal resurfacing procedures.
- On February 17, 2022, the Company announced the submission of a 510(k) premarket notification (“510(k) submission”) to the U.S. Food and Drug Administration, which is intended to obtain a general indication for use of the Renuvion Dermal handpiece in dermatological procedures requiring ablation and resurfacing of the skin.
- On March 14, 2022, the Company announced it had been notified by the U.S. Food and Drug Administration (“FDA”; “Agency”) that the Agency intended to post a Medical Device Safety Communication related to the Company’s Advanced Energy products. The Safety Communication was subsequently posted by the FDA on March 14, 2022.
Highlights & Developments Subsequent to Quarter End:
- On April 4, 2022, the Company announced it had submitted a 510(k) premarket notification to the U.S. Food and Drug Administration. The 510(k) submission is intended to expand its general indication for the Renuvion APR Handpiece to include a specific indication for use in subcutaneous dermatological and aesthetic procedures to achieve thermal coagulation/contraction to improve the appearance of lax (loose) skin in the neck and submental region.
Management Comments:
“We are pleased to report total revenue results that exceeded the high-end of the growth expectations we provided on our fourth quarter earnings call,” said Charlie Goodwin, President and Chief Executive Officer. “Our Advanced Energy sales growth of 41% year-over-year was fueled primarily by global handpiece sales, which increased more than 80% year-over-year, with global generator sales increasing 2% year-over-year. As expected, generator sales to new customers were negatively impacted following the FDA Safety Communication in mid-March, however global demand for our Renuvion handpieces has remained strong.”
Mr. Goodwin continued: “Our Advanced Energy products remain on the market with their existing FDA 510(k) clearances, and we continue to market and sell them for their existing clinical indications, while advancing our regulatory strategy to pursue clearance for new specific indications. We have updated our outlook for total revenue in fiscal 2022 based on our better-than-expected results in the first quarter, and our growth expectations for the second quarter and second half of 2022. We look forward to continued engagement with the FDA in support of our three pending 510(k) premarket notifications, which remain under review by the Agency.”
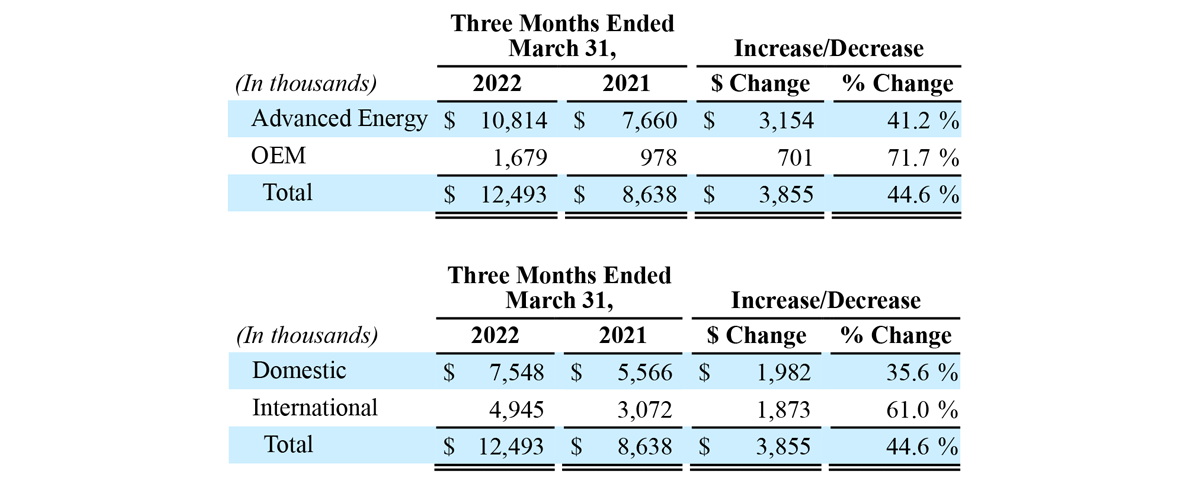
The following tables present revenue by reportable segment and geography:
First Quarter 2022 Results:
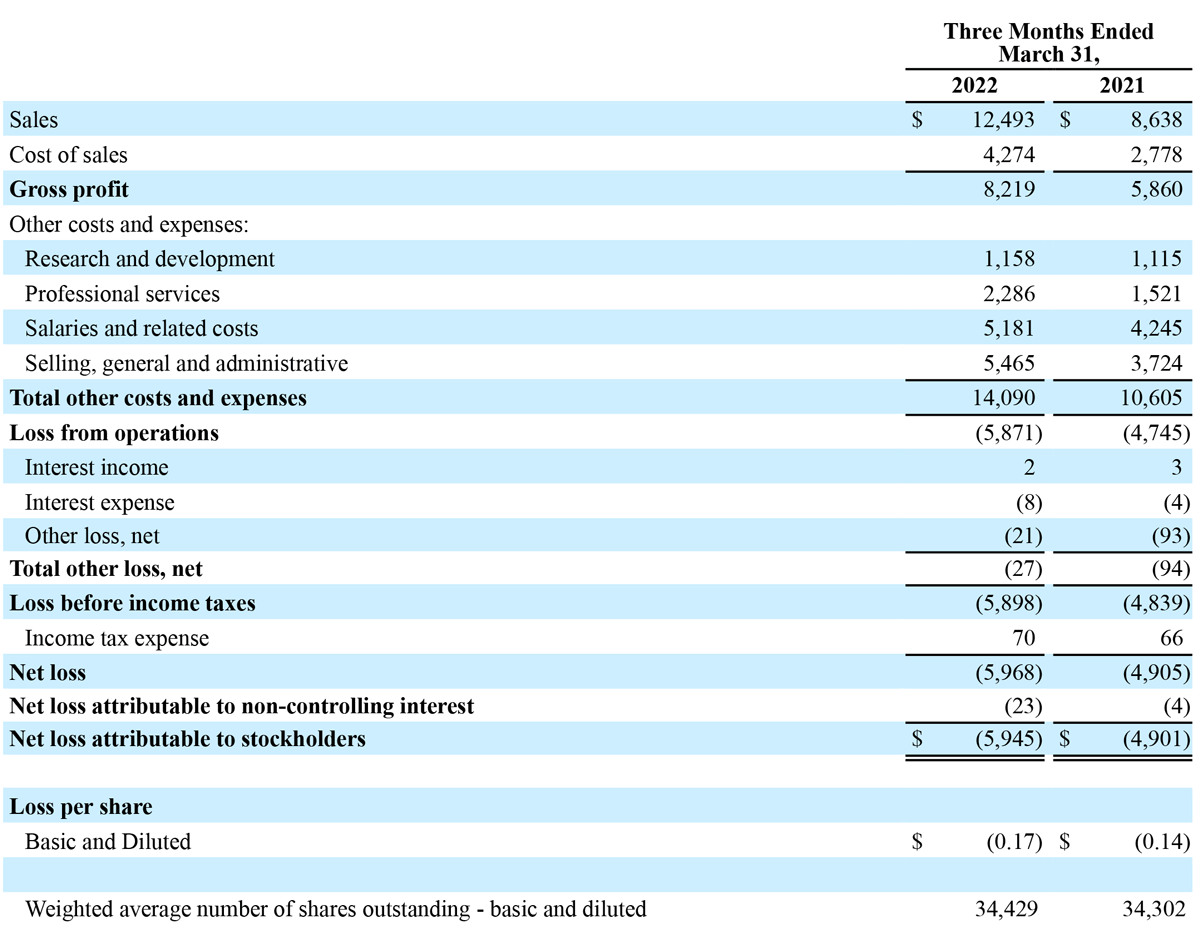
Total revenue for the three months ended March 31, 2022 increased $3.9 million, or 45% year-over-year, to $12.5 million, compared to $8.6 million in the prior year period. Advanced Energy segment sales increased $3.2 million, or 41% year-over-year, to $10.8 million, compared to $7.7 million in the prior year period. OEM segment sales increased $0.7 million, or 72% year-over-year to $1.7 million, compared to $1.0 million in the prior year period. For the first quarter of 2022, revenue in the United States increased $2.0 million, or 36% year-over-year, to $7.5 million, and international revenue increased $1.9 million, or 61% year-over-year, to $4.9 million. In the first quarter of 2022, Advanced Energy revenue growth was driven by an increase in global, utilization-based demand for the Company’s handpieces and adoption of its generator technology in international markets. This increase was partially offset by a decrease in the adoption of the Company’s generator technology in the U.S. following the FDA Safety Communication on March 14, 2022. OEM revenue growth was driven by increased sales volume to existing customers, including sales to Symmetry Surgical under our 10-year generator manufacturing and supply agreement.
Gross profit for the three months ended March 31, 2022, increased $2.4 million, or 40% year-over-year, to $8.2 million, compared to $5.9 million in the prior year period. Gross margin for the three months ended March 31, 2022, was 66%, compared to 68% for the prior year period. The decrease in profit margins for the three months ended March 31, 2022 from the prior year period was primarily attributable to sales mix between the Company’s two segments, with the OEM segment comprising a higher percentage of total sales, along with product and geographic mix within the Advanced Energy segment, and higher costs to manufacture inventory as the Company continues to experience higher shipping costs. These decreases were partially offset by the mix of newer product models as the Company obtains registrations in various markets, allowing these products to be introduced into the markets it serves.
Operating expenses for the three months ended March 31, 2022 increased $3.5 million, or 33% year-over-year, to $14.1 million, compared to $10.6 million for the prior year period. The year-over-year change in operating expenses was driven by a $1.7 million increase in selling, general and administrative expenses, a $0.9 million increase in salaries and related costs and a $0.8 million increase in professional services.
Income tax expense for the three months ended March 31, 2022 was $0.1 million, consistent with the prior year period.
Net loss attributable to stockholders for the three months ended March 31, 2022 was $5.9 million, or $0.17 per share, compared to a net loss of $4.9 million, or $0.14 per share, for the prior year period.
Full Year 2022 Financial Outlook:
The Company is updating financial guidance for the year ending December 31, 2022 to:
- Total revenue in the range of $52.5 million to $59.0 million, representing growth of 8% to 22% year-over-year, compared to total revenue of $48.5 million for the year ended December 31, 2021. The Company’s prior guidance range for total revenue was $50.0 million to $63.0 million, representing growth of 3% to 30% year-over-year.
- Total revenue guidance assumes:
- Advanced Energy revenue in the range of approximately $46.0 million to $52.0 million, representing growth of 7% to 21% year-over-year, compared to Advanced Energy revenue of $43.0 million for the year ended December 31, 2021. The Company’s prior guidance range for Advanced Energy revenue was $43.5 million to $56.0 million, representing growth of 1% to 30% year-over-year.
- The Advanced Energy revenue range reflects potential negative impacts in the U.S. on new customer adoption, and on procedure-related demand for handpieces, as a result of the FDA Safety Communication on March 14, 2022.
- The Advanced Energy revenue range continues to assume contributions from the initial commercial launches for new specific clinical indications for dermal resurfacing and skin laxity procedures, based on the Company’s target of receiving FDA 510(k) clearances related to these procedures by the end of the third quarter of 2022.
- The Advanced Energy revenue range continues to assume that international growth is driven by demand in existing international markets.
- OEM revenue in the range of $6.5 million to $7.0 million, which is unchanged from the Company’s prior guidance, representing growth of 18% to 27% year-over-year, compared to $5.5 million for the year ended December 31, 2021.
- Advanced Energy revenue in the range of approximately $46.0 million to $52.0 million, representing growth of 7% to 21% year-over-year, compared to Advanced Energy revenue of $43.0 million for the year ended December 31, 2021. The Company’s prior guidance range for Advanced Energy revenue was $43.5 million to $56.0 million, representing growth of 1% to 30% year-over-year.
- Total revenue guidance assumes:
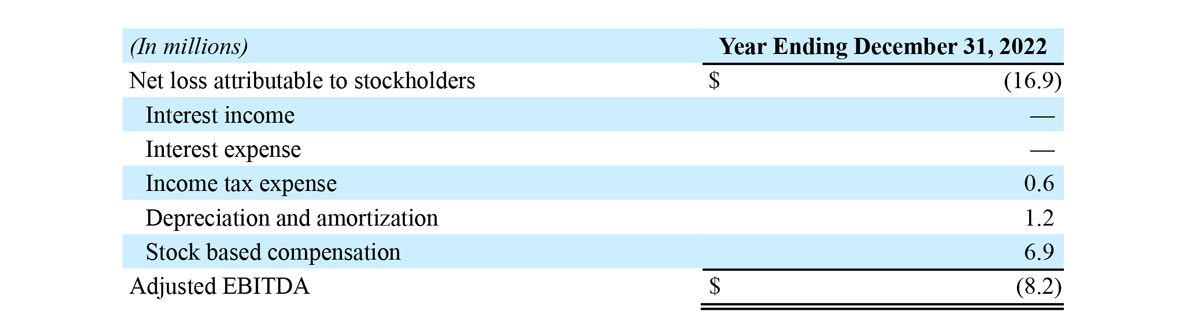
- Net loss attributable to stockholders in the range of $19.0 million to $14.7 million, compared to net loss attributable to stockholders of $15.2 million for the year ended December 31, 2021. The Company’s prior guidance range for net loss attributable to stockholders was $21.1 million to $12.1 million.
- Adjusted EBITDA loss in the range of $10.1 million to $6.4 million, compared to adjusted EBITDA loss of $8.8 million for the year ended December 31, 2021. The Company’s prior guidance range for Adjusted EBITDA loss was $12.3 million to $3.0 million.
Conference Call Details:
Management will host a conference call at 8:00 a.m. Eastern Time on May 12, 2022 to discuss the results of the quarter and to host a question and answer session. To listen to the call by phone, interested parties may dial 877-407-8289 (or 201-689-8341 for international callers) and provide access code 13728514. Participants should ask for the Apyx Medical Corporation Call. A live webcast of the call will be accessible via the Investor Relations section of the Company’s website and at:
https://services.choruscall.com/mediaframe/webcast.html?webcastid=OHGg8TE0
A telephonic replay will be available approximately two hours after the end of the call through May 26, 2022. The replay can be accessed by dialing 877-660-6853 for U.S. callers or 201-612-7415 for international callers and using the replay access code: 13728514. The webcast will be archived on the Investor Relations section of the Company’s website.
Investor Relations Contact:
ICR Westwicke on behalf of Apyx Medical Corporation
Mike Piccinino, CFA
investor.relations@apyxmedical.com
About Apyx Medical Corporation:
Apyx Medical Corporation is an advanced energy technology company with a passion for elevating people’s lives through innovative products, including its Helium Plasma Technology products marketed and sold as Renuvion and J-Plasma in surgical markets. Renuvion and J-Plasma offer surgeons a unique ability to provide controlled heat to tissue to achieve their desired results. The Company also leverages its deep expertise and decades of experience in unique waveforms through OEM agreements with other medical device manufacturers. For further information about the Company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Cautionary Statement on Forward-Looking Statements:
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including but not limited to, any statements regarding the potential impact of the COVID-19 pandemic and the actions by governments, businesses and individuals in response to the situation; projections of net revenue, margins, expenses, net earnings, net earnings per share, or other financial items; projections or assumptions concerning the possible receipt by the Company of any regulatory approvals from any government agency or instrumentality including but not limited to the U.S. Food and Drug Administration, supply chain disruptions, component shortages, manufacturing disruptions or logistics challenges; or macroeconomic or geopolitical matters and the impact of those matters on the Company’s financial performance.
Forward-looking statements and information are subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause the Company’s actual results to differ materially and that could impact the Company and the statements contained in this release include but are not limited to risks, uncertainties and assumptions relating to the regulatory environment in which the Company is subject to, including the Company’s ability to gain requisite approvals for its products from the U.S. Food and Drug Administration and other governmental and regulatory bodies, both domestically and internationally; the impact of the recent FDA Safety Communication on our business and operations; factors relating to the effects of the COVID-19 pandemic; sudden or extreme volatility in commodity prices and availability, including supply chain disruptions; changes in general economic, business or demographic conditions or trends; changes in and effects of the geopolitical environment; liabilities and costs which the Company may incur from pending or threatened litigations, claims, disputes or investigations; and other risks that are described in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021 and the Company’s other filings with the Securities and Exchange Commission. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
APYX MEDICAL CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
(Unaudited)
APYX MEDICAL CORPORATION
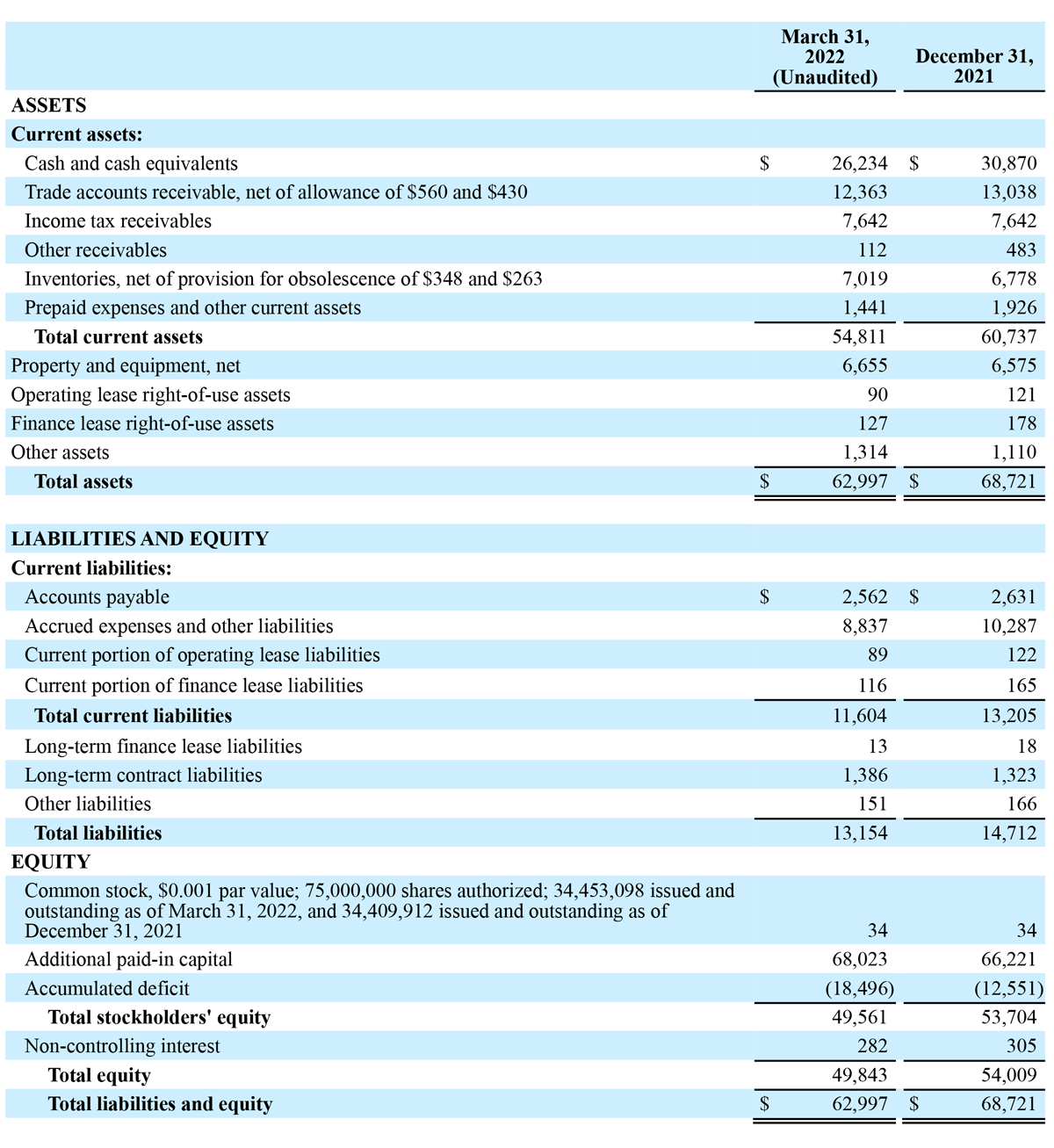
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
APYX MEDICAL CORPORATION
RECONCILIATION OF GAAP NET LOSS RESULTS TO NON-GAAP ADJUSTED EBITDA
(Unaudited)
Use of Non-GAAP Financial Measure
We present the following non-GAAP measure because we believe such measure is a useful indicator of our operating performance. Our management uses this non-GAAP measure principally as a measure of our operating performance and believes that this measure is useful to investors because it is frequently used by analysts, investors and other interested parties to evaluate companies in our industry. We also believe that this measure is useful to our management and investors as a measure of comparative operating performance from period to period. The non-GAAP financial measure presented in this release should not be considered as a substitute for, or preferable to, the measures of financial performance prepared in accordance with GAAP.
The Company has presented the following non-GAAP financial measure in this press release: adjusted EBITDA. The Company defines adjusted EBITDA as its reported net income (loss) attributable to stockholders (GAAP) plus income tax expense (benefit), interest, depreciation and amortization, and stock-based compensation expense.
The following unaudited table presents a reconciliation of net loss attributable to stockholders to Adjusted EBITDA loss for the year ending December 31, 2022. The reconciliation assumes the mid-point of the Adjusted EBITDA loss range and the midpoint of each component of the reconciliation, corresponding to guidance for GAAP net loss attributable to stockholders of $19.0 million to $14.7 million for the year ending December 31, 2022.