APYX MEDICAL CORPORATION REPORTS FOURTH QUARTER AND FULL YEAR 2023 FINANCIAL RESULTS; INTRODUCES FULL YEAR 2024 FINANCIAL OUTLOOK
Advanced Energy Sales increased 15% year-over-year and 23% quarter-over-quarter in the fourth quarter; increased 18% year-over-year in 2023
CLEARWATER, FL — March 21, 2024 – Apyx® Medical Corporation (NASDAQ:APYX) (“Apyx Medical;” the “Company”), the manufacturer of a proprietary helium plasma and radiofrequency technology marketed and sold as Renuvion®, today reported financial results for its fourth quarter and full year ended December 31, 2023, and introduced financial expectations for the full year ending December 31, 2024.
Fourth Quarter 2023 Financial Summary:
- Total revenue of $14.7 million, an increase of 16% year-over-year.
- Advanced Energy revenue of $12.1 million, an increase of 15% year-over-year.
- OEM revenue of $2.5 million, an increase of 22% year-over-year.
- Net loss attributable to stockholders of $9.6 million, an increase of $3.6 million, or 59%, year-over-year.
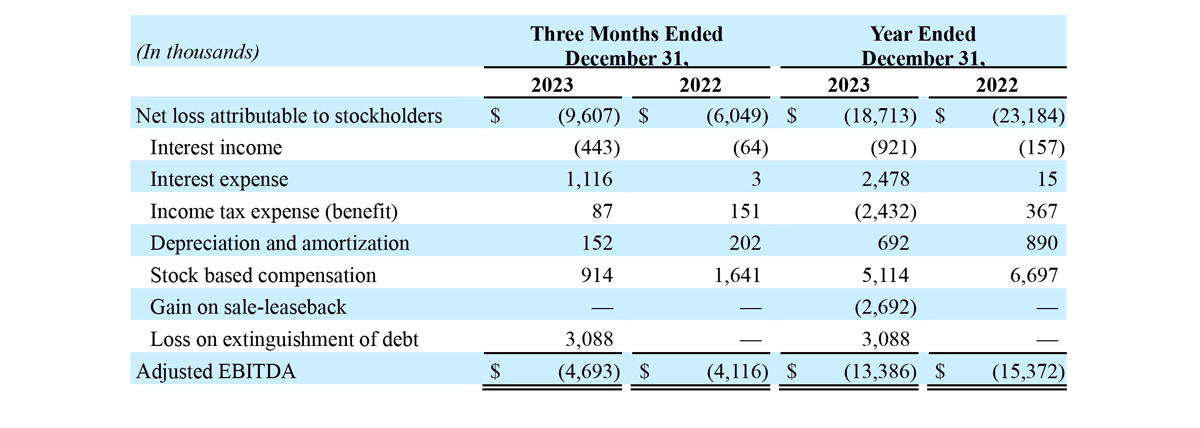
- Adjusted EBITDA loss of $4.7 million, an increase of $0.6 million, or 14%, year-over-year.
Full Year 2023 Financial Summary:
- Total revenue of $52.3 million, an increase of 18% year-over-year.
- Advanced Energy revenue of $43.4 million, an increase of 18% year-over-year.
- OEM revenue of $9.0 million, an increase of 16% year-over-year.
- Net loss attributable to stockholders of $18.7 million, a decrease of $4.5 million, or 19%, year-over-year.
- Adjusted EBITDA loss of $13.4 million, a decrease of $2.0 million, or 13%, year-over-year.
Fourth Quarter 2023 Operating Summary:
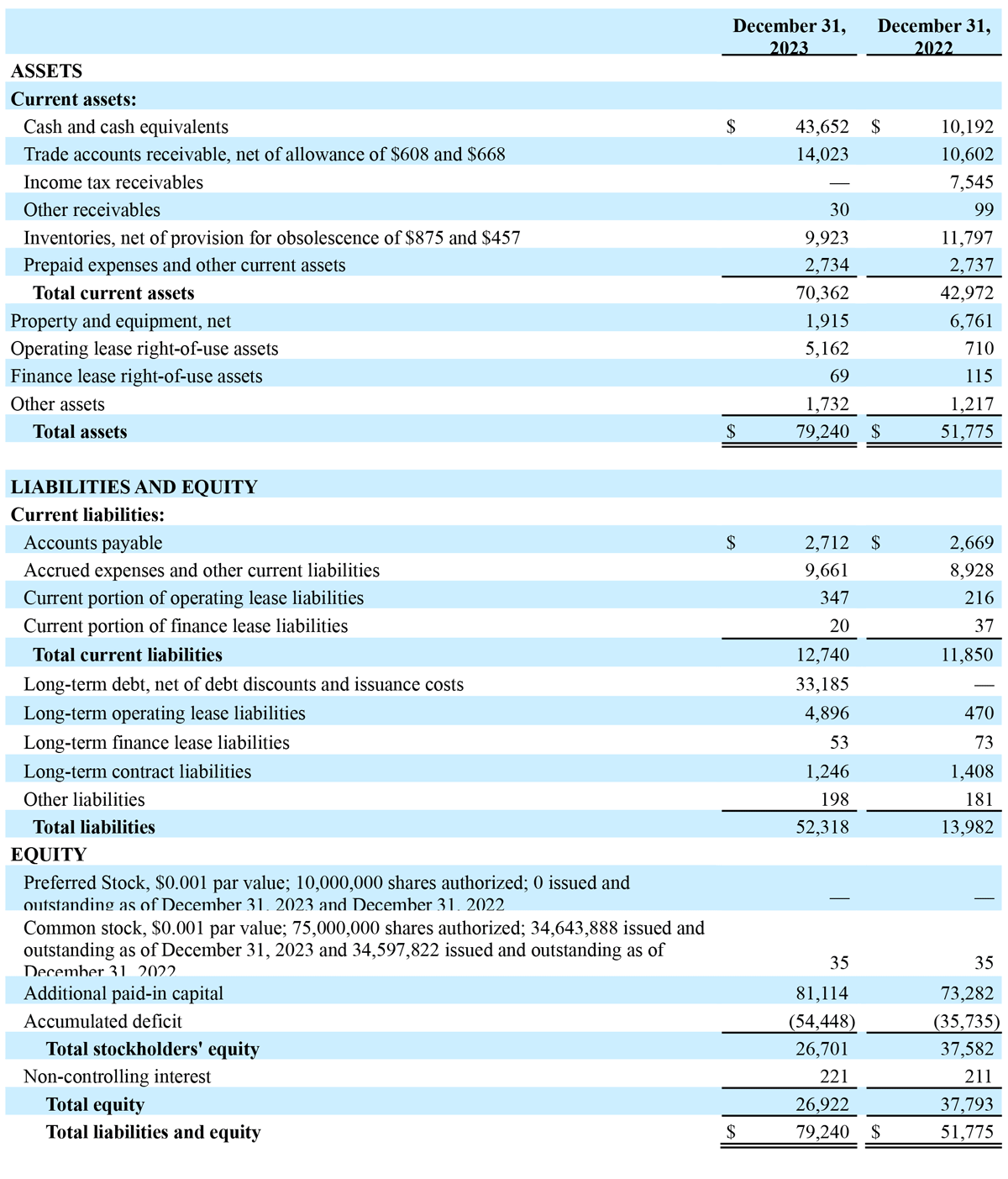
- On November 9, 2023, the Company announced a new, five-year credit agreement with Perceptive Credit Holdings IV, LP (“Perceptive”), an affiliate of Perceptive Advisors. The credit agreement provides for a facility of up to $45.0 million consisting of senior, secured term loans. The Perceptive credit facility matures on November 8, 2028 and includes an initial loan of $37.5 million and a delayed draw loan of $7.5 million. The initial loan of $37.5 million was fully funded on November 8, 2023, with approximately $11.0 million of the proceeds used to satisfy all obligations under the Company’s MidCap credit agreement, as well as approximately $2.7 million of transaction fees and other expenses related to the transactions.
- On November 28, 2023, the Company announced that it had appointed Matthew Hill as its Chief Financial Officer, effective December 4, 2023.
Management Comments:
“We generated double-digit growth in sales of our Advanced Energy products in the fourth quarter, driven by growth in global generator and handpiece sales of more than 25% and 10% year-over-year, respectively,” said Charlie Goodwin, President and Chief Executive Officer. “As previously reported, our Advanced Energy revenue fell short of our expectations, as the capital equipment purchasing environment proved to be more challenging than anticipated. Ultimately, we were pleased to return to double-digit revenue growth on a full year basis in 2023. In addition, we reduced our annual net loss attributable to stockholders and Adjusted EBITDA loss by 19% and 13%, respectively, and delivered a significant reduction in cash used in operations this year.”
Mr. Goodwin continued: “Our 2024 guidance assumes Advanced Energy generator demand in the cosmetic surgery market will continue to be impacted, as prospective surgeon customers remain reticent to purchase new capital equipment, offset fully or partially by growth in handpiece sales this year. We intend to continue managing our resources efficiently while investing strategically, and with $44 million in cash and equivalents at year-end, we have the requisite capital to execute our initiatives. Our team remains focused on leveraging the strong progress we made in 2023, which enabled us to secure new 510(k) clearances for specific clinical indications, introduce our Renuvion Apyx One Generator and Micro Handpiece in the U.S., and expand awareness of the safety and efficacy of our technology. With this progress in view, we believe Apyx Medical is poised to capitalize on the unique capabilities of our Renuvion technology – and favorable long-term tailwinds in our industry – to target sustainable and profitable growth in the future.”
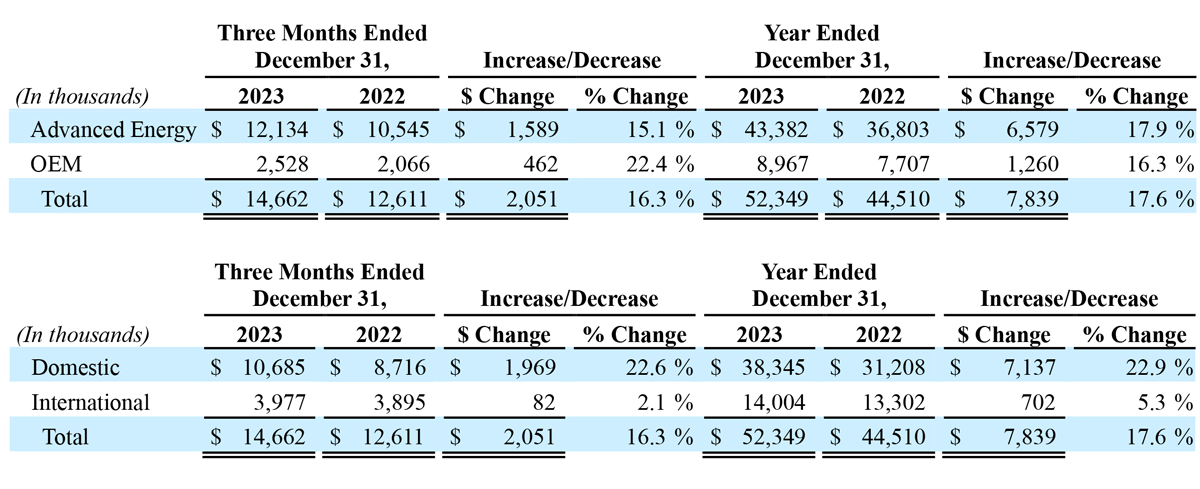
The following tables present revenue by reportable segment and geography:
 Fourth Quarter 2023 Results:
Fourth Quarter 2023 Results:
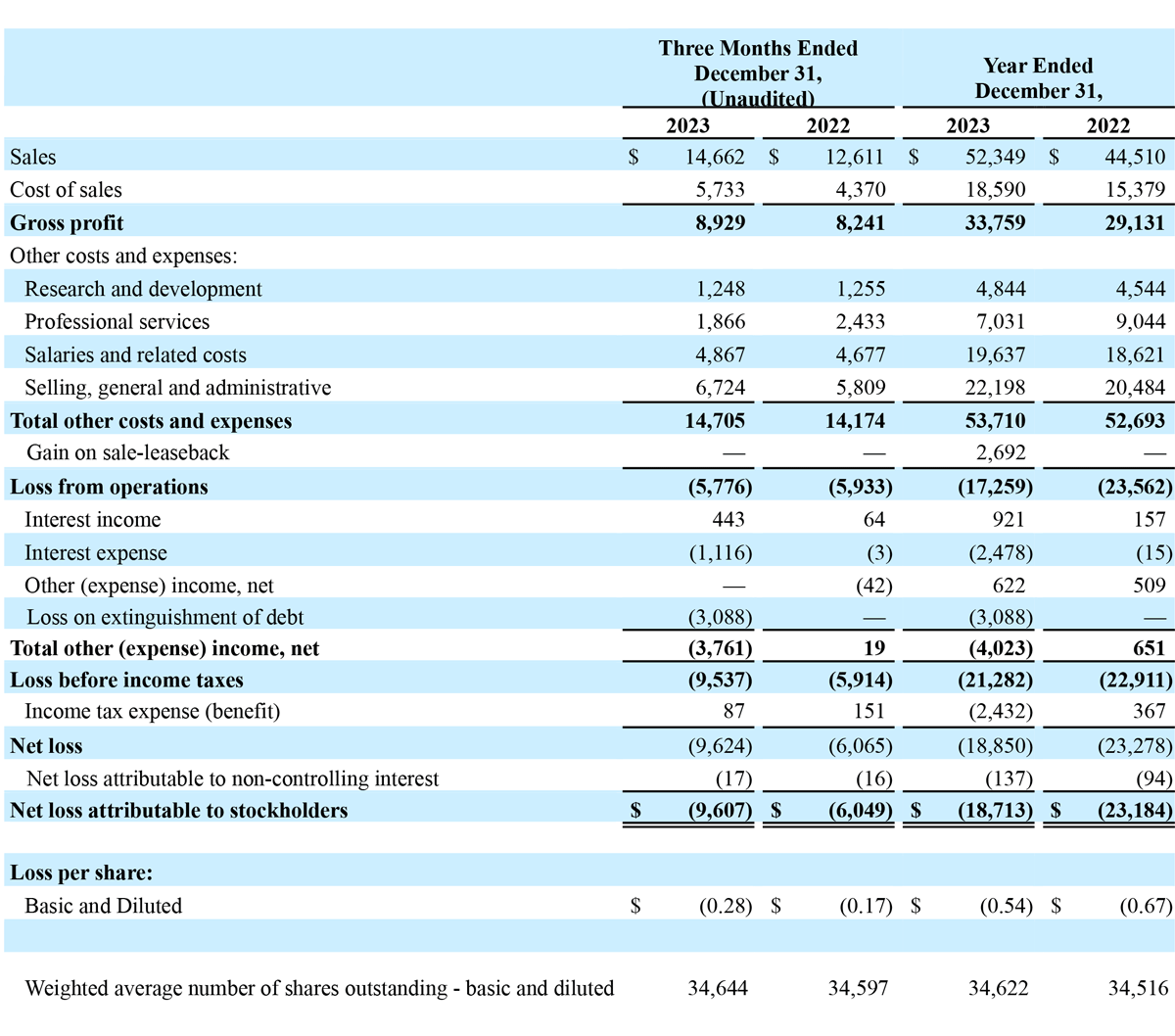
Total revenue for the three months ended December 31, 2023, increased $2.1 million, or 16% year-over-year, to $14.7 million, compared to $12.6 million in the prior year period. Advanced Energy segment revenue increased $1.6 million, or 15% year-over-year, to $12.1 million. OEM segment revenue increased $0.5 million, or 22% year-over-year, to $2.5 million, compared to $2.1 million in the prior year period. The increase in Advanced Energy revenue was primarily due to growth in domestic sales, driven by increased sales of single-use handpieces, as well as sales of the Company’s new Apyx One Console (launched in January 2023) to existing customers. Advanced Energy revenue also benefited from growth in international markets, driven by an increase in generator sales. The increase in OEM segment revenue was due to increased sales volume to existing customers. Domestic revenue increased $2.0 million, or 23% year-over-year, to $10.7 million, and international revenue increased $0.1 million, or 2% year-over-year, to $4.0 million.
Gross profit for the three months ended December 31, 2023, increased $0.7 million, or 8% year-over-year, to $8.9 million, compared to $8.2 million in the prior year period. Gross profit margin for the three months ended December 31, 2023, was 60.9%, compared to 65.3% in the prior year period. The decrease in gross profit margins was primarily attributable to changes in product mix within the Company’s Advanced Energy segment, customer mix, higher material and inbound shipping costs to manufacture inventory and additional reserves on inventories as a result of lower than expected sales.
Operating expenses for the three months ended December 31, 2023, increased $0.5 million, or 4% year-over-year, to $14.7 million, compared to $14.2 million in the prior year period. The increase in operating expenses was driven by a $0.9 million increase in selling, general and administrative expenses and a $0.2 million increase in salaries and related costs. These increases were partially offset by a $0.6 million decrease in professional services expenses.
Other (expense) income, net for the three months ended December 31, 2023, and 2022 was $(3.8) million and $19 thousand, respectively. The change was driven primarily by a $3.1 million expense related to the extinguishment of the Company’s prior Credit Agreement, as well as increased interest expense compared to the prior year period.
Income tax expense for the three months ended December 31, 2023 and 2022 was $0.1 million and $0.2 million, respectively.
Net loss attributable to stockholders for the three months ended December 31, 2023, was $9.6 million, or $0.28 per share, compared to $6.0 million, or $0.17 per share, in the prior year period.
Adjusted EBITDA loss for the three months ended December 31, 2023 and 2022 was $4.7 million and $4.1 million, respectively.
Full Year 2023 Results:
Total revenue for the year ended December 31, 2023, increased $7.8 million, or 18%, to $52.3 million, compared to $44.5 million in 2022. Advanced Energy segment revenue increased $6.6 million, or 18% year-over-year, to $43.4 million, compared to $36.8 million in 2022. OEM segment revenue increased $1.3 million, or 16% year-over-year, to $9.0 million, compared to $7.7 million in 2022. The increase in Advanced Energy revenue was primarily due to growth in domestic sales, which was driven primarily by generator sales of the Company’s new Apyx One Console (launched in January 2023) to existing customers, as well as increased sales of single-use handpieces. Advanced Energy revenue also benefited from growth in international sales, driven by an increase in sales of generators. The increase in OEM revenue was due to increased sales volume to existing customers, as well as incremental new sales upon the commencement of the supply arrangement related to the completion of the development portion of some of the Company’s OEM development agreements. Domestic revenue increased $7.1 million, or 23% year-over-year, to $38.3 million, and international revenue increased $0.7 million, or 5% year-over-year, to $14.0 million.
Net loss attributable to stockholders for the year ended December 31, 2023, was $18.7 million, or $0.54 per share, compared to $23.2 million, or $0.67 per share, in the prior year.
Full Year 2024 Financial Outlook:
The Company is introducing its financial guidance for the year ending December 31, 2024:
- Total revenue in the range of $49.7 million to $52.9 million, representing a decrease of approximately 5% to growth of approximately 1% year-over-year, compared to total revenue of $52.3 million for the year ended December 31, 2023.
- Total revenue guidance assumes:
- Advanced Energy revenue in the range of $41.6 million to $44.6 million, representing a decrease of approximately 4% to growth of approximately 3% year-over-year, compared to Advanced Energy revenue of $43.4 million for the year ended December 31, 2023.
- OEM revenue in the range of $8.1 million to $8.3 million, representing a decrease of 10% to 7% year-over-year, compared to $9.0 million for the year ended December 31, 2023.
- Total revenue guidance assumes:
- Net loss attributable to stockholders of approximately $26.5 million to $24.3 million, compared to $18.7 million for the year ended December 31, 2023.
Conference Call Details:
Management will host a conference call at 8:00 a.m. Eastern Time on March 21, 2024 to discuss the results of the quarter and year, and to host a question and answer session. To listen to the call by phone, interested parties may dial 877-407-8289 (or 201-689-8341 for international callers) and provide access code 13744178. Participants should ask for the Apyx Medical Corporation call. A live webcast of the call will be accessible via the Investor Relations section of the Company’s website and at:
https://event.choruscall.com/mediaframe/webcast.html?webcastid=KtFzwD3M
A telephonic replay will be available approximately three hours after the end of the call through the following two weeks. The replay can be accessed by dialing 877-660-6853 for U.S. callers or 201-612-7415 for international callers and using the replay access code: 13744178. The webcast will be archived on the Investor Relations section of the Company’s website.
Investor Relations Contact:
ICR Westwicke on behalf of Apyx Medical Corporation
Mike Piccinino, CFA
investor.relations@apyxmedical.com
About Apyx Medical Corporation:
Apyx Medical Corporation is an advanced energy technology company with a passion for elevating people’s lives through innovative products, including its Helium Plasma Technology products marketed and sold as Renuvion in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion and J-Plasma offer surgeons a unique ability to provide controlled heat to tissue to achieve their desired results. The Company also leverages its deep expertise and decades of experience in unique waveforms through OEM agreements with other medical device manufacturers. For further information about the Company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Cautionary Statement on Forward-Looking Statements:
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including but not limited to, projections of net revenue, margins, expenses, net earnings, net earnings per share, or other financial items; projections or assumptions concerning the possible receipt by the Company of any regulatory approvals from any government agency or instrumentality including but not limited to the U.S. Food and Drug Administration (the “FDA”), supply chain disruptions, component shortages, manufacturing disruptions or logistics challenges; or macroeconomic or geopolitical matters and the impact of those matters on the Company’s financial performance.
Forward-looking statements and information are subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause the Company’s actual results to differ materially and that could impact the Company and the statements contained in this release include but are not limited to risks, uncertainties and assumptions relating to the regulatory environment in which the Company is subject to, including the Company’s ability to gain requisite approvals for its products from the FDA and other governmental and regulatory bodies, both domestically and internationally; the impact of the March 14, 2022 FDA Safety Communication on our business and operations; sudden or extreme volatility in commodity prices and availability, including supply chain disruptions; changes in general economic, business or demographic conditions or trends; changes in and effects of the geopolitical environment; liabilities and costs which the Company may incur from pending or threatened litigations, claims, disputes or investigations; and other risks that are described in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and the Company’s other filings with the Securities and Exchange Commission. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
APYX MEDICAL CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
APYX MEDICAL CORPORATION
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
APYX MEDICAL CORPORATION
RECONCILIATION OF GAAP NET LOSS RESULTS TO NON-GAAP ADJUSTED EBITDA
(Unaudited)
Use of Non-GAAP Financial Measure:
We present the following non-GAAP measure because we believe such measure is a useful indicator of our operating performance. Our management uses this non-GAAP measure principally as a measure of our operating performance and believes that this measure is useful to investors because it is frequently used by analysts, investors and other interested parties to evaluate companies in our industry. We also believe that this measure is useful to our management and investors as a measure of comparative operating performance from period to period. The non-GAAP financial measure presented in this release should not be considered as a substitute for, or preferable to, the measures of financial performance prepared in accordance with GAAP.
The Company has presented the following non-GAAP financial measure in this press release: adjusted EBITDA. The Company defines adjusted EBITDA as its reported net income (loss) attributable to stockholders (GAAP) plus income tax expense (benefit), interest, depreciation and amortization, stock-based compensation expense and other significant non-recurring items.