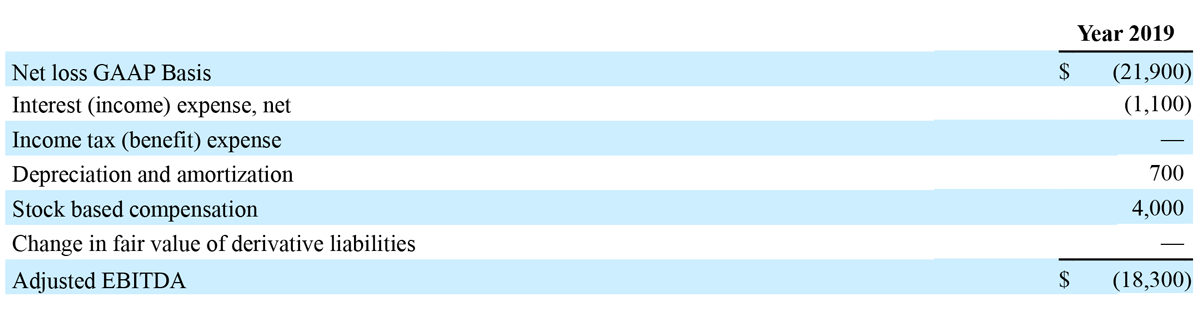APYX MEDICAL CORPORATION REPORTS SECOND QUARTER AND FIRST HALF OF 2019 FINANCIAL RESULTS AND UPDATES FISCAL YEAR 2019 OUTLOOK
Advanced Energy Sales of $5.3 million in Q2, up 69% year-over-year
CLEARWATER, FL — AUGUST 7, 2019 – Apyx™ Medical Corporation (NASDAQ:APYX) (the “Company”), a maker of medical devices and supplies and the developer of Helium Plasma Technology, marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market, today reported financial results for its second quarter ended June 30, 2019.
Second Quarter 2019 Financial Summary:
- Total Q2 revenue from continuing operations of $6.6 million, up 78% year-over-year.
- Advanced Energy revenue of $5.3 million, up 69% year-over-year.
- OEM revenue of $1.3 million, up 125% year-over-year.
- Total Q2 GAAP net loss from continuing operations of $4.3 million versus total GAAP net loss from continuing operations of $2.9 million for the second quarter of 2018.
- Total Q2 adjusted EBITDA loss from continuing operations of $3.7 million versus adjusted EBITDA loss from continuing operations of $2.4 million for 2018.
Highlights Subsequent to Quarter End:
- On July 1, 2019, the Company announced it has been added to the Russell 3000® and Russell 2000® The Company’s inclusion in the indexes occurred as part of the annual reconstitution by FTSE Russell of its U.S. equity indexes and became effective on July 1st.
- On July 15, 2019, the Company announced the appointment of four members to its Medical Advisory Board: Dr. Brian M. Kinney, Dr. Paul G. Ruff, Dr. Richard D. Gentile and Dr. Edward M. Zimmerman.
- On July 31, 2019, the Company announced it has enhanced its clinical and regulatory affairs teams with the appointment of Kari Larson and Libet Garber, Ph.D. to the positions of Senior Director of Clinical Affairs and Director of Global Regulatory Affairs, respectively.
- On August 5, 2019, the company announced it had received U.S. Food and Drug Administration (FDA) 510(k) clearance to market and sell its next-generation J-Plasma Precise Handpiece.
- On August 6, 2019, the Company announced it had appointed Minnie Baylor-Henry to its Board of Directors, effective August 1, 2019, where she will be chairing the newly formed Regulatory and Compliance Committee. Ms. Baylor-Henry brings with her more than 20 years of recognized leadership in Regulatory Affairs, including leadership positions at Johnson & Johnson and the U.S. Food and Drug Administration.
Management Comments:
“Apyx Medical is excited to report another quarter of strong revenue growth driven by global demand for our Renuvion generators and handpieces in the cosmetic surgery market,” said Charlie Goodwin, President and Chief Executive Officer. “I am particularly pleased with our operating and financial performance in light of the challenges we faced during the second quarter, which was made possible by the hard work and dedication of our organization. During the quarter, we continued to execute on our strategic growth objectives to increase our share of the $1.5 billion U.S. cosmetic surgery market, penetrate existing international markets and expand into new countries with strong demand from cosmetic surgeons for our highly differentiated Renuvion technology. Importantly, we also made excellent progress in our pursuit of new clinical indications for Renuvion in the U.S., with the submission in late July of an investigational device exemption, or IDE, application for a new study evaluating the use of Renuvion in dermal resurfacing procedures. We remain committed to taking a methodical approach to obtaining new clinical indications for our Renuvion technology, supported by the strategic insight and efforts of our recently enhanced clinical and regulatory affairs teams.”
Mr. Goodwin continued: “We are raising our 2019 revenue, net loss and adjusted EBITDA loss guidance range based upon the stronger than expected financial results that we have achieved in the second quarter and outlook for the rest of 2019. As we enter the second half of 2019, we will continue to pursue our strategic initiatives and make targeted investments in order to deliver strong, sustained growth and profitability in the years to come. Looking ahead, with truly innovative technologies, a solid balance sheet and an entire organization dedicated to supporting our surgeon customers and improving patient outcomes, we remain convinced that Apyx Medical is uniquely positioned to reshape the cosmetic surgery market, while delivering attractive returns for our shareholders.”
Second Quarter 2019 Results:
The following table represents revenue from continuing operations by reportable segment:
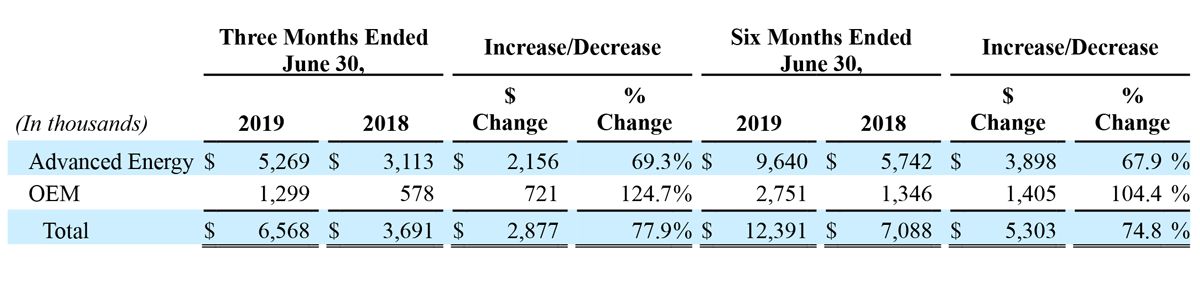
Total revenue from continuing operations for second quarter 2019 increased $2.9 million, or 77.9%, to $6.6 million, compared to $3.7 million in the second quarter of 2018. Sales of the Company’s Advanced Energy generators and handpieces drove the increase in total revenue in second quarter 2019, with OEM segment sales contributing modestly to the year-over-year increase in total revenue from continuing operations during the second quarter 2019 period. Advanced Energy segment sales increased $2.2 million, or 69.3% year-over-year, to $5.3 million, compared to $3.1 million last year. OEM segment sales increased $0.7 million, or 124.7% year-over-year, to $1.3 million, compared to $0.6 million last year.
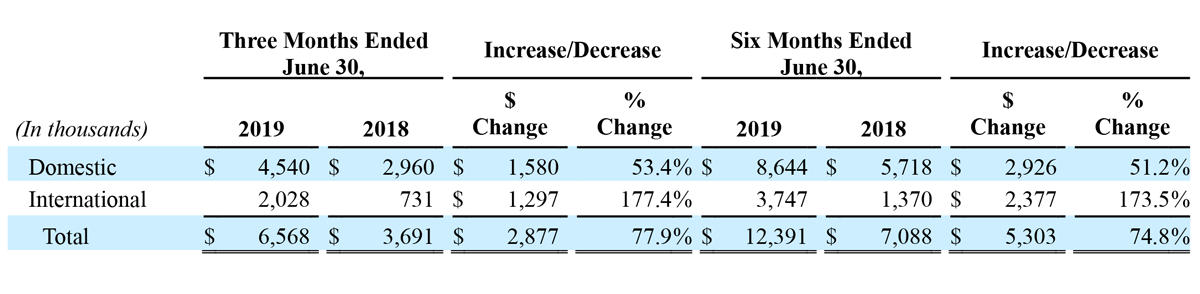
For the second quarter 2019, revenue from continuing operations in the United States increased $1.6 million, or 53.4% year-over-year, to $4.5 million, and international revenue from continuing operations increased $1.3 million, or 177.4% year-over-year, to $2.0 million. International sales growth in the second quarter was primarily driven by sales to international distributors in the Company’s Advanced Energy segment.
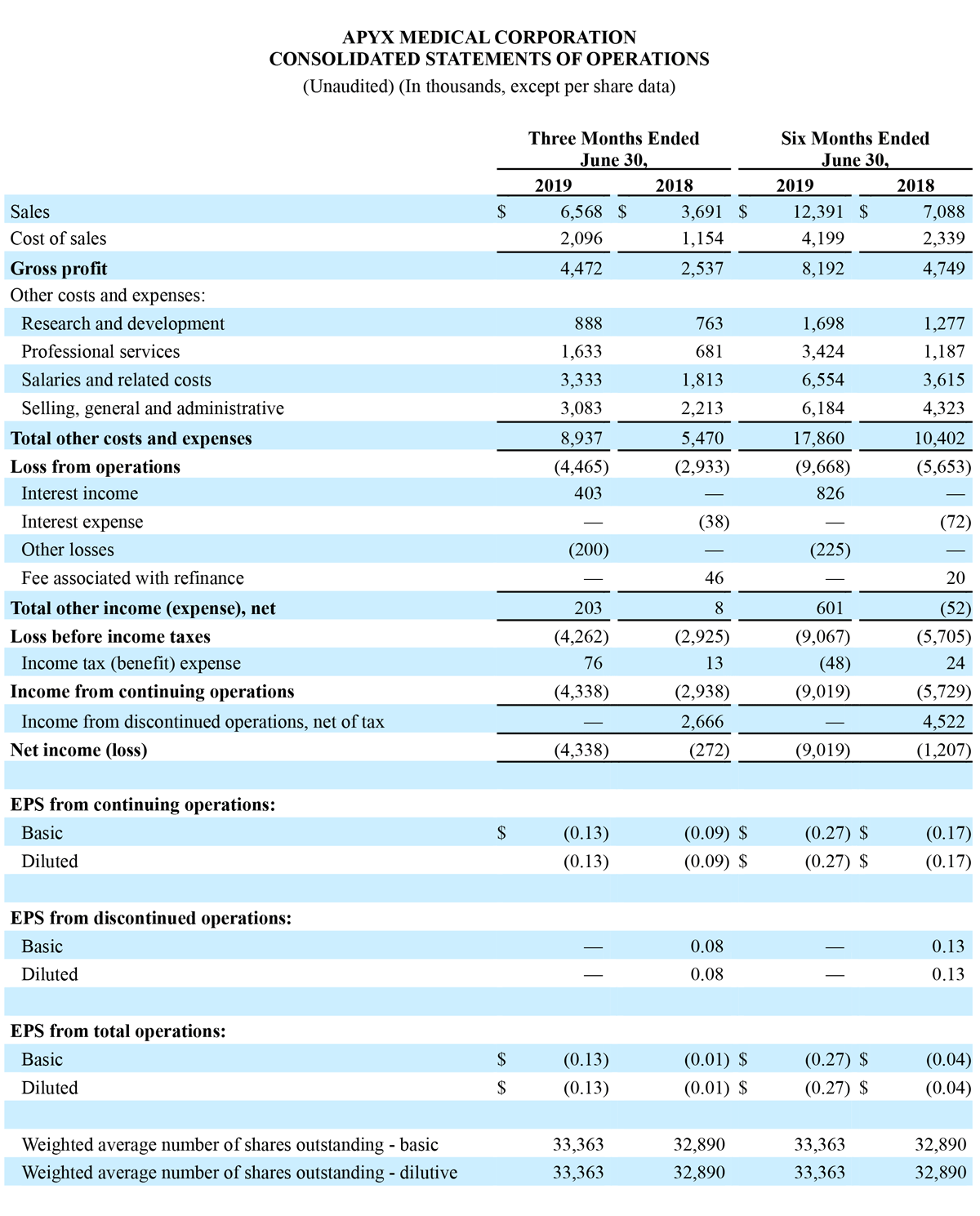
Gross profit for the second quarter of 2019 increased $1.9 million, or 76.3% year-over-year, to $4.5 million, compared to $2.5 million for second quarter of 2018. Gross margin for the second quarter of 2019 was 68.1%, compared to 68.7% last year. The primary drivers of the decrease in gross profit margin were Advanced Energy product mix and Advanced Energy sales outside the U.S., which represented a higher mix of total sales in the second quarter of 2019 compared to last year. OEM gross margins were lower in the second quarter of 2019 when compared to the prior year period, driven primarily by revenue related to our new Product, Manufacturing, and Supply agreements with Symmetry, which did not contribute to revenue results in the prior period.
Operating expenses from continuing operations for the second quarter of 2019 increased $3.5 million, or 63.4% year-over-year, to $8.9 million, compared to $5.5 million for the second quarter of 2018. The year-over-year change in operating expenses from continuing operations was primarily driven by a $1.5 million increase in salaries and related costs, a $1.0 million increase in professional services costs, a $0.9 million increase in selling, general, and administration, and a $0.1 million increase in research and development expenses.
Net loss from continuing operations for second quarter 2019 was $(4.3) million, or $(0.13) per diluted share, compared to a net loss from continuing operations of $(2.9) million, or $(0.09) per diluted share, for the second quarter of 2018. Total income from discontinued operations, net of tax, was $2.7 million in the second quarter of 2018.
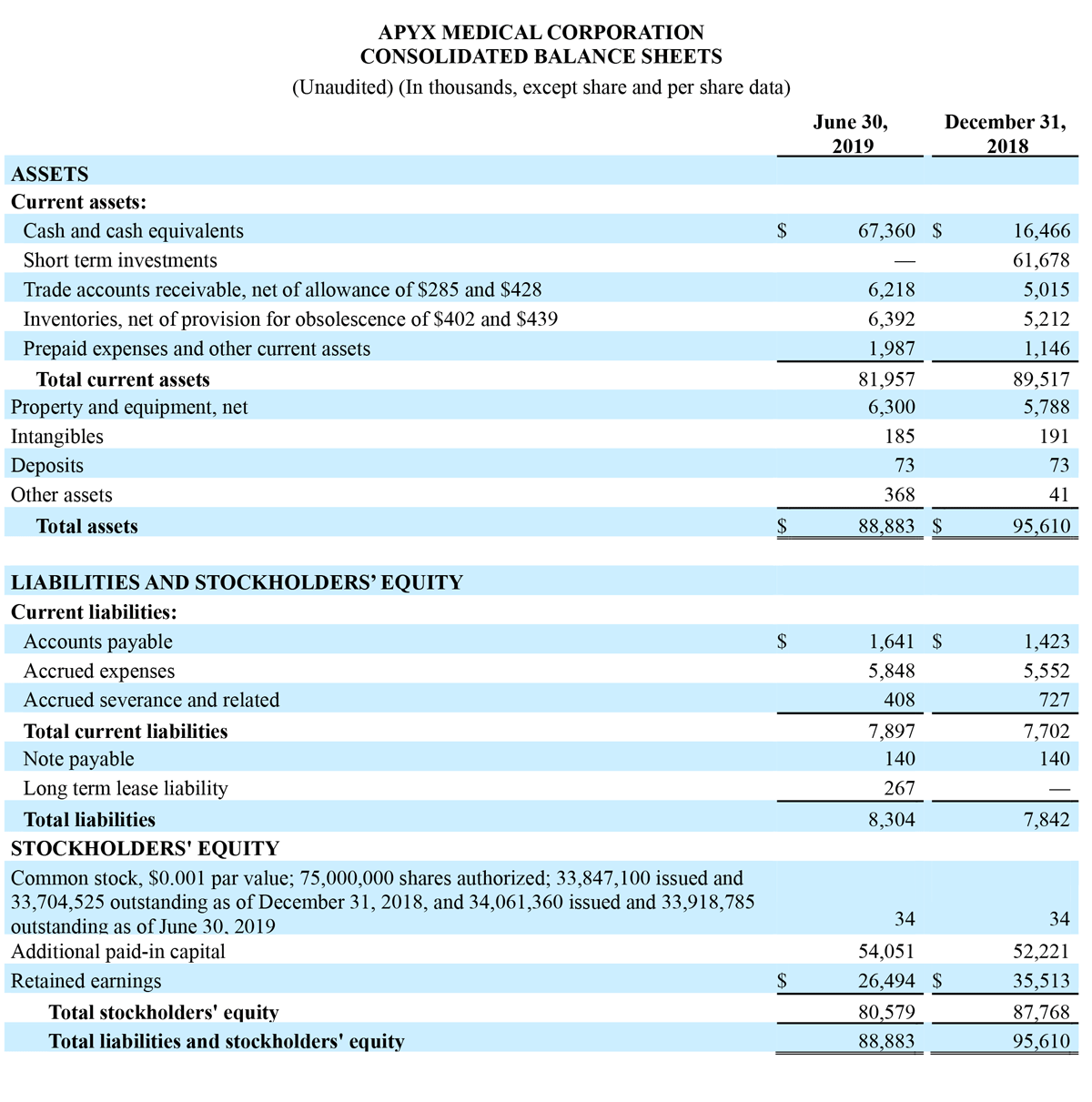
As of June 30, 2019, the Company had cash and equivalents of $67.4 million as compared to cash and equivalents of $16.5 million and short-term investments of $61.7 million as of December 31, 2018. The Company had working capital of $74.1 million as of June 30, 2019 as compared to $81.8 million as of December 31, 2018.
First Half of 2019 Results:
Total revenue for the six months ended June 30, 2019 increased $5.3 million, or 74.8%, to $12.4 million, compared to $7.1 million in the six months ended June 30, 2018. Total revenue growth was driven by a 67.9% increase in Advance Energy sales and a 104.4% increase in OEM sales.
Net loss from continuing operations for the six months ended June 30, 2019 was $(9.0) million, or $(0.27) per diluted share, compared to a loss from continuing operations of $(5.7) million, or $(0.17) per diluted share, for the six months ended June 30, 2018.
2019 Financial Outlook:
The Company is updating its fiscal year 2019 financial guidance:
- Total revenue in the range of $26.5 million to $27.5 million, representing growth of 59% to 65% year-over-year, compared to total revenue from continuing operations of $16.7 million in fiscal year 2018. The Company’s prior guidance range for total revenue was $25.5 million to $26.5 million, representing growth of 53% to 59% year-over-year.
- Total revenue guidance assumes:
- Advanced Energy revenue in the range of $21.5 million to $22.5 million, representing growth of 65% to 72% year-over-year, compared to Advanced Energy revenue of $13.1 million in fiscal year 2018. The Company’s prior guidance range for Advanced Energy revenue was $20.5 million to $21.5 million, representing growth of 57% to 65% year-over-year.
- The Company continues to expect OEM revenue of $5.0 million, representing growth of 38% year-over-year, compared to $3.6 million for fiscal year 2018.
- GAAP net loss in the range of $22.4 million to $21.4 million, compared to GAAP net loss from continuing operations of $9.5 million in fiscal year 2018. The Company’s prior guidance range for GAAP net loss was $23.5 million to $22.5 million.
- Adjusted EBITDA loss in the range of $18.8 million to $17.8 million, compared to adjusted EBITDA loss from continuing operations of $11.7 million in fiscal year 2018. The Company’s prior guidance range for Adjusted EBITDA loss was $19.9 million to $18.9 million.
- Total revenue guidance assumes:
Conference Call Details:
Management will host a conference call at 4:30 p.m. Eastern Time on August 7 to discuss the results of the quarter and to host a question and answer session. To listen to the call by phone, interested parties may dial 844-507-6493 (or 647-253-8641 for international callers) and provide access code 5115769. Participants should ask for the Apyx Medical Corporation Call. A live webcast of the call will be accessible via the Investor Relations section of the Company’s website and at:
https://event.on24.com/wcc/r/2017153/8BF3360A6C8B7308D4E8C83EACC0EF15
A telephonic replay will be available approximately two hours after the end of the call through August 21, 2019. The replay can be accessed by dialing 800-585-8367 for U.S. callers or 416-621-4642 for international callers and using the replay access code: 5115769. The webcast will be archived on the Investor Relations section of the Company’s website.
Investor Relations Contact:
Westwicke Partners on behalf of Apyx Medical Corporation
Mike Piccinino, CFA
investor.relations@apyxmedical.com
About Apyx Medical Corporation:
Apyx Medical Corporation (formerly Bovie® Medical Corporation) is an advanced energy technology company with a passion for elevating people’s lives through innovative products in the cosmetic and surgical markets. Known for its innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients it serves. The company’s Helium Plasma Technology is marketed and sold as Renuvion in the cosmetic surgery market and J-Plasma in the hospital surgical market. Renuvion offers plastic surgeons, fascial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma system allows surgeons to operate with a high level of precision and virtually eliminating unintended tissue trauma. The Company also leverages its deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the Company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Cautionary Statement on Forward-Looking Statements:
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company’s ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this release can be found in the Company’s filings with the Securities and Exchange Commission including the Company’s Report on Form 10-K for the year ended December 31, 2018 and subsequent Form 10-Q filings. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
APYX MEDICAL CORPORATION
RECONCILIATION OF GAAP NET INCOME/(LOSS) RESULTS TO NON-GAAP ADJUSTED EBITDA/(LOSS)
(Unaudited) (In thousands)
Use of Non-GAAP Financial Measures
We present these non-GAAP measures because we believe these measures are useful indicators of our operating performance. Our management uses these non-GAAP measures principally as a measure of our operating performance and believes that these measures are useful to investors because they are frequently used by analysts, investors and other interested parties to evaluate companies in our industry. We also believe that these measures are useful to our management and investors as a measure of comparative operating performance from period to period.
The Company has presented the following non-GAAP financial measures in this press release: adjusted EBITDA. The Company defines adjusted EBITDA as its reported net income/(loss) (GAAP) plus income tax expense, interest, depreciation and amortization, stock-compensation expense, and changes in value of derivative liabilities.
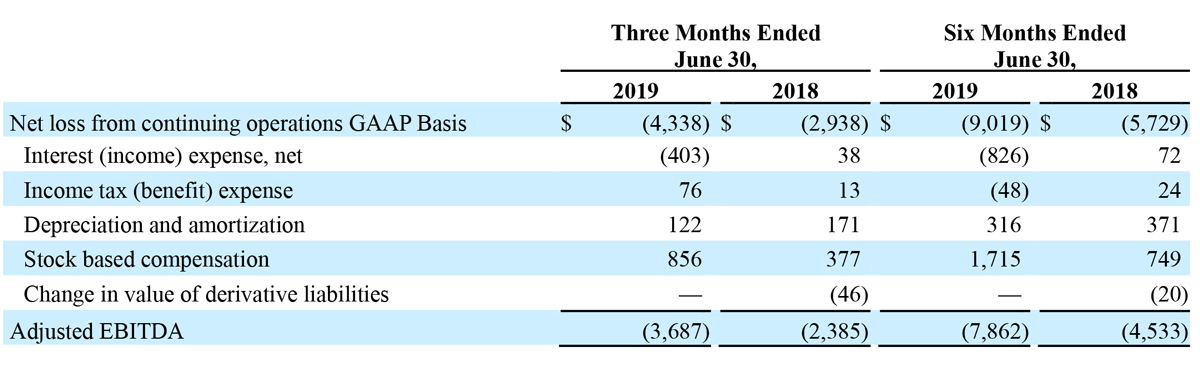
The following unaudited table presents a reconciliation of net loss to Adjusted EBITDA for our 2019 guidance. The reconciliation assumes the mid-point of the Adjusted EBITDA loss range and the midpoint of each component of the reconciliation, corresponding to guidance for GAAP net loss of $22.4 million to $21.4 million for 2019.